|
年间契约型资讯服务
商品编码
1396216
前 1000+ 家生物设施指数和生物製造商资料库Top 1000+ Bio-Facility Index and Biomanufacturers Subscription Database |
||||||
该资料库是唯一追踪全球超过 1,500 个生物製药工厂的生物设施资料库。 大多数受调查的机构生产商业和临床产品,设备和耗材的销售额超过 100 亿美元。 资料库内容自 2008 年以来每季更新一次,包含全面的资讯和见解。 它也基于生物製药和生命科学产业30年的评估和基准。 您的订阅包括季度通讯和综合年度回顾。
1000+生物製药设施指数透过对每个设施进行计数、索引和排名,对生物製药生产区域和集群按区域进行定量分析。 包含多个栏位用于分析和比较,包括:
- 生物反应器的总升数
- 重组蛋白和其他培养产生的蛋白质、抗体、疫苗和细胞产品
- 与生物製造相关的就业
- 未来的生物製造能力
- 用于商业和临床用途的生物製剂数量
如何利用TOP1000BIO.COM
瞭解生物製品的生产地对于该行业及其供应商来说非常重要。 製造业涉及高成本以及对当地社区和就业基地的长期承诺。 此数据可用于以下目的:
- 设施位置:基于优秀员工的集中度:减少就业挑战
- 分配销售区域:决定在哪些区域以及如何配备人员:确定销售潜力
- 房地产评估:基于每个设施的 "吸引力点" (按区域和次区域)
- 人力资源/招募:建立可行的集群需要受过教育且经验丰富的人员。
- 企业和投资者:地区发展机构和政府可以使用此资源来定义就业集群。
- 产业成长:产业成长最快的地方是哪里?
- 新兴地区中国、印度和俄罗斯等发展中国家的设施如何? 成长速度有多快?
- 公司排名:公司在就业、产能、整体集中度的整体排名如何?
The only bio-facilities database subscription that tracks over 1,500+ facilities worldwide manufacturing biopharmaceuticals. Hundreds of facilities with commercial products, many more supporting clinical production, account for well over $10 billion in equipment and consumable sales. Updated quarterly since 2008 and includes comprehensive information and insights. Based on 30 years of evaluating and benchmarking the biopharmaceutical and life sciences industry. Subscription includes quarterly newsletter, and comprehensive annual review.
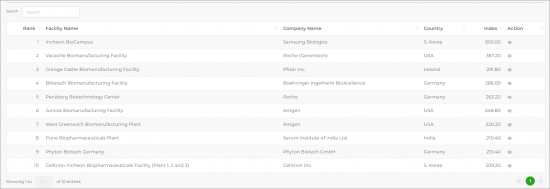
SAMPLE VIEW
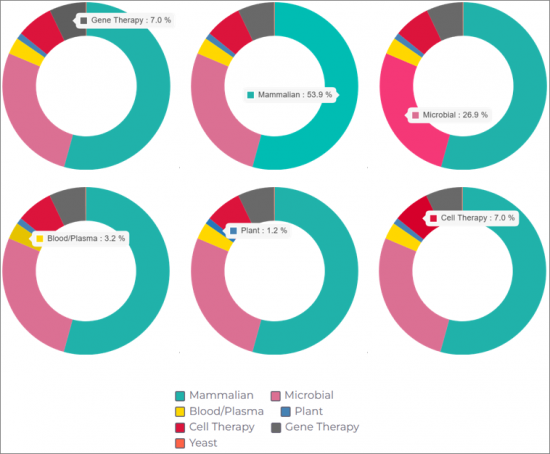
LITERS CAPACITY BY PLATFORM/SYSTEM

BIOPLAN'S TOP 1000 GLOBAL BIOPHARMACEUTICAL FACILITIES INDEX™

‘Top 1000+ Biopharmaceutical Facilities Index counts, indexes and ranks each facility’, provides regional quantitative analysis of biomanufacturing regions and clusters. Multiple fields included for analysis and comparative review, including:
- Overall total bioreactor liters
- Recombinant and other culture-produced proteins, antibodies, vaccines, cellular products
- Biomanufacturing-related employment
- Upcoming Biomanufacturing future capacity
- Number of commercial and clinical biological products
APPLICATIONS FOR THE TOP1000BIO.COM
Knowing where biologics are manufactured is important to the industry and its suppliers. Manufacturing involves high cost, long-term commitments to the community and employment base. This data can be used for:
- Facility siting: Based on concentration of qualified employees; reduces hiring challenges
- Sales territory allocations: Determine where and how to staff territories; defines expectations for sales
- Real estate valuation: For facilities, based on regional and sub-region 'quality points for attractiveness
- HR and Recruiters: Look for a critical mass of educated and skilled staff is needed to establish a viable cluster
- Companies, investors: Regional development authorities & governments use the resource to define employment clusters
- Industry Growth: Where is the industry growing most rapidly, and by which indexed factors?
- Emerging Regions: Facilities in China, India, Russia, other developing regions? How rapidly are they growing?
- Company Ranking: How do companies rank overall, in employment, capacity and overall concentration?