|
年间契约型资讯服务
商品编码
1254616
治疗性 mRNA 的专利追踪Therapeutic mRNA Patent Monitor |
|||||||
服务的主要特点
每个季度我们都会为您提供最新的Excel数据库,包括:
- 新专利申请
- 新授予的专利
- 过期或放弃的专利
- 知识产权转让(再转让、许可)、知识产权联合研究
- 专利诉讼/异议
- 按技术类别、应用和治疗领域分类的专利:mRNA 设计、递送、製造、基于 mRNA 的疫苗、基于 mRNA 的疗法、传染病、肿瘤疾病和其他疾病
- 更新在线数据库的超链接(法律状态、文檔等)
每个季度我们都会提供一份 PDF 报告,其中包含:
- 主要季度业绩和数据
- 涵盖专利格局变化的图表和评论
- 主要知识产权公司和新进入者
IP Analyst Access(每年 100 小时):
- 与知识产权分析师就 mRNA 治疗领域的趋势、分析、特定专利技术和公司知识产权组合进行问答会议和讨论
释放 mRNA 疗法的优势:季度专利跟踪服务
针对 COVID-19 大流行的 mRNA 疫苗的成功凸显了基于 mRNA 的治疗技术的破坏性方面。 COVID-19 是 mRNA 技术平台的众多适应症之一,对广泛的微生物病原体和癌症的新疫苗具有很高的前景。 此外,治疗性 mRNA 的开发不仅用于疫苗接种,还用于治疗罕见和常见疾病,如遗传、纤维化和自身免疫性疾病。 然而,要将 mRNA 确立为一种通用的治疗工具,仍然存在一系列挑战。 为了克服这些挑战,已经开发了各种新技术,包括优化 mRNA 运输的方法、具有组织嗜性的载体和 mRNA 积累。 此外,mRNA 疫苗和治疗剂的发展路径在几个重要方面存在差异,例如 mRNA 免疫原性、组织嗜性、递送和药代动力学,因此未来的发展方向可能会因治疗应用而异。
了解不同公司在这个瞬息万变的环境中的知识产权地位和战略至关重要。 这些知识有助于我们发现商业风险和机遇,确定新的研究领域,并了解我们竞争对手的战略。
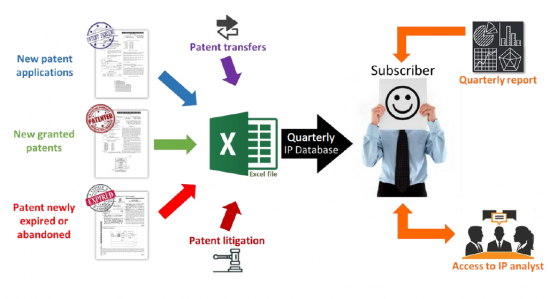
Therapeutic mRNA Patent Tracking Service 利用季度更新的 excel 文件从季度分析报告和与分析师的直接互动中获益。 excel 文件包含新的专利申请、新获得的专利、专利到期/放弃、专利转让(再转让、许可)和专利诉讼/异议。 这些专利按应用和治疗领域以及 mRNA 设计(自扩增和环状 RNA)、载体、製造和储存过程等技术领域进行分组。 这个方便的 Excel 电子表格具有指向更新的在线数据库的超链接,允许您按多个标准进行搜索,包括优先权日期、专利受让人、专利法律状态和特定领域。 我们的季度 PDF 报告详细介绍了过去三个月的知识产权趋势,重点介绍了主要的知识产权公司和专利技术。 直接访问分析师提供按需问答环节,并就治疗性 mRNA 领域的季度结果、趋势、分析、特定专利技术或公司专利组合进行公开讨论。
专利跟踪服务的优势
了解竞争对手的知识产权活动和未来意图
通过使用专利跟踪服务,您可以了解当前竞争对手的专利活动、知识产权动态、收购和许可等专利转让、专利诉讼、技术开发、研发战略等。 此外,有可能在早期发现公司业务领域的新进入者。
了解最新技术趋势,预测技术趋势
通过跟踪最近的专利申请,您可以跟踪该领域的最新创新。 了解有关专利发明的更多信息并跟踪技术发展。 新的技术解决方案可以激发和改进您的研发活动。
防止可能损害业务的知识产权註册
您可以在专有权被授予之前获得有关专利申请的信息,并及时做出回应,以防止可能对您的业务产生不利影响的知识产权註册增加。
快速应对侵权行为,降低法律风险
通过监控新颁发的专利,您可以定期评估您的操作自由度以及您的产品或工艺是否不受专利保护以及是否有他人拥有的有效知识。您可以确保製造、销售是安全的并在不侵犯任何所有权的情况下使用。
利用免费技术降低研发项目的风险
通过跟踪过期和废弃的专利,您可以识别已进入公共领域且可以安全开发的发明。
了解当前的知识产权趋势和竞争对手的知识产权战略
每个季度,我们都会提供过去三个月的 IP 趋势,详细介绍主要 IP 参与者、新进入者和关键专利技术。 重点专利申请人及其发明、块专利、有前途的专利以及新到期或放弃的重点专利被突出显示。
拜访知识产权分析师
通过电话或电子邮件直接与分析师互动,通过按需问答环节和讨论(每年 100 小时)获取有关特定专利技术和公司 IP 组合的具体意见。

Key features of the service
Every quarter an up-to-date Excel database including:
- New patent applications
- Patent applications newly granted
- Expired or abandoned patents
- Transfer of IP rights (re-assignment, licensing) and IP collaborations.
- Patent litigations and oppositions
- Patents categorized by technological segmentation, application and therapeutic area: mRNA design, delivery, manufacturing, mRNA-based vaccines, mRNA-based therapeutics, infectious diseases, oncological disorders and other diseases.
- Hyperlinks to updated online database (legal status, documents etc.)
Every quarter a PDF report including:
- Key facts & figures of the quarter
- Graphs and comments covering the patent landscape evolutions
- A close look at the key IP players and newcomers
Access to an IP analyst for 100 hours per year:
- Q&A sessions and discussions with our IP analysts regarding trends, analyses, specific patented technologies, or companies' IP portfolios in the field of mRNA therapeutics.
Unlocking the benefits of mRNA therapeutics: A quarterly patent monitoring service
The success of mRNA vaccines as a response to the COVID-19 pandemic has shone a light on the disruptive aspect of mRNA-based therapeutic technology. COVID-19 is one of many indications for the mRNA technology platform, which holds high promise for new vaccines with a wide spectrum of microbial pathogens and cancers. Moreover, therapeutic mRNA is not limited to vaccination, but has also been developed to treat both rare and common diseases (genetic diseases, fibrotic diseases, autoimmune disorders, etc.). However, a series of challenges remain to be addressed before mRNA can be established as a general therapeutic modality. An array of new technologies is being developed to surmount these challenges, including approaches to optimize mRNA cargos, carriers with inherent tissue tropism, and mRNA storing, for example. The orientation of future development might also depend on the therapeutic application, as the development pathway for mRNA vaccines and therapeutics differs in several important respects, such as mRNA immunogenicity, tissue tropism, delivery, or pharmacokinetics.
In this fast-evolving context, it is crucial to understand the intellectual property position and strategy of the different players. Such knowledge can help detect business risks and opportunities, identify emerging research areas, and understand competitors' strategies.

The mRNA Therapeutics Patent Monitoring Service allows you to take advantage of a quarterly-updated Excel file and benefit from both quarterly analysis reports and direct interaction with our analysts. The Excel file includes new patent applications, new granted patents, patents expired/abandoned, patent transfers (re-assignment, licensing), and patent litigation/opposition. The patents are categorized by technology segments, including mRNA design (self-amplifying and circular RNA), carriers, manufacturing, and storing process, but also by application and therapeutic area. This useful Excel spreadsheet with hyperlinks to updated online database allows for multi-criteria searches, including priority date, patent assignees, legal status of patents, and specific segments. The quarterly PDF reports provide the IP trends over the last three months, with a close look at key IP players and patented technologies. Direct access to our analysts offers you on-demand Q&A sessions and open discussions regarding the quarterly report results, trends, analyses, specific patented technologies, or companies' patent portfolios in the field of Therapeutic mRNA.
Benefits of the patent monitoring service
Keep an eye on your competitors' IP activities and their future intentions.
With the help of the patent monitoring service, you will be aware of your competitors' current patenting activities, their IP dynamics, patent transfers including acquisitions and licenses, patent litigation, technology development, and R&D strategies. You will also be able to detect newcomers early in your business area.
Keep track of the latest technology developments and stay ahead of technology trends.
By taking note of any recent patent filings, you can track the latest innovations in the field. You will get details on claimed inventions and you can follow technology developments. New technical solutions could inspire and improve your R&D activity.
Prevent registration of IP rights that may be harmful to your business.
You will obtain information on patent applications filed even before exclusive rights have been granted, and you can react in time to prevent registration of IP rights that may be harmful to your business.
React quickly to infringements and mitigate legal risks.
Monitoring newly-issued patents allows you to regularly assess your freedom-to-operate, ensuring that your products or processes are not covered by patents and thus can be manufactured, sold, or used safely without infringing valid IP rights owned by others.
Take advantage of free technologies and reduce R&D project risks.
By tracking both expired patents and abandoned patents, you will be able to identify inventions entering the public domain that you can use safely for your development.
Understand the current IP trends and IP strategy of competitors.
On a quarterly basis, the report will provide the IP trends over the last three months, with a close look at key IP players, newcomers, and key patented technologies. Main patent applicants and their inventions, blocking patents, promising patents, and key newly expired or abandoned patents will be highlighted.
Access an IP analyst.
Take advantage of direct interaction with our analysts by phone and/or email and get specific input for specific patented technologies and companies' IP portfolios through on-demand Q&A sessions and discussion (100 hours per year).












