 |
年间契约型资讯服务
商品编码
1349829
HER2 低转移性乳癌市场:Tumor DeckHER2-Low Metastatic Breast Cancer - Tumour Deck |
||||||
HER2 表现量低(定义为 HER2 IHC 评分 1+ 或 2+)且未检测到 ERBB2 扩增的肿瘤被归类为 HER2 低转移性乳癌类别。 这是新定义的 HER2 阴性 BC 子集,其 HER2 免疫组织化学 (IHC) 评分为 1+ 或评分 2+/原位杂交 (ISH) 阴性表型。 IHC/ISH 是目前用于定义 HER2 表达的唯一标准技术。
针对 HER2 低表达的转移性乳癌的治疗正在迅速发展。 最近的临床试验表明,CDK4/6 抑制剂与内分泌治疗联合作为标准一线治疗是有效的。 此外,PI3K抑制剂和AKT抑制剂的使用也在临床试验中进行研究,可能在不久的将来提供进一步的治疗选择。
HER2低表达转移性乳癌是一种新的乳癌亚型,约占新诊断乳癌病例的50%至60%。 这显示HER2低表现的转移性乳癌是一种相对常见的亚型。 HER2 表达低的乳癌通常被视为 HER2 阴性,即使它们表达 HER2。 儘管 HER2 低表达在 HR+ 乳癌中更为常见,但研究表明,在 HR 阴性乳癌中也观察到这种情况。
目前,HER2低表达转移性乳癌的主要治疗方法是化疗、内分泌治疗、标靶治疗等不同治疗方法的结合。
近年来,标靶治疗在临床试验中显示出前景,并被视为替代疗法。 由于标靶治疗的最新进展,目前 HER2 低转移性乳癌的护理标准正在迅速发展。 最近的临床试验表明,新型 HER2 导向的抗体药物偶联物 (ADC) 在治疗 HER2 低肿瘤中具有显着的临床益处。 其中一种核准的 ADC 曲妥珠单抗 deruxtecan (T-Dxd) 在 HER2 低表达乳癌中显示出有希望的结果。
除了标靶治疗外,内分泌治疗也是HER2低表现乳癌的重要治疗选择,尤其是荷尔蒙受体阳性患者。 合併治疗,例如CDK4/6抑制剂和内分泌治疗的合併治疗,也有望改善HER2低表现乳癌患者的预后。
本报告调查了全球 HER2 低转移性乳癌市场,并提供了市场现状、病例量趋势、患者趋势、竞争产品市场定位和市场机会。
目录
第 1 章执行摘要
第2章 HER2低表现转移性乳癌概述
- HER2低表达转移性乳癌的定义、症状、病因和发病机制
- HER2-低状态的临床意义
第3章 HER2低表现转移性乳癌的定义与诊断
第 4 章 HER2 低表现转移性乳癌的流行病学
- 按国家/地区划分的盛行率
第5章HER2低表现转移性乳癌的治疗实务
- 目前的治疗方法
- 处理演算法
- 用于快速核准的可接受端点
第 6 章核准的 HER2 低表达转移性乳癌标靶治疗
- 已核准的治疗方法摘要
第7章管道临床试验
- HER2低表达转移性乳癌管线状况概述与分析
- HER2低表现转移性乳癌的竞争格局
- 关键分子及临床试验结果
- 主要药物核准与上市的时间表
第8章三期资产
- 临床试验和结果
第9章二期资产
- 临床试验和结果
第10章第一期资产
- 临床试验和结果
第 11 章 HER2 低表现转移性乳癌管线中的非临床分子
第12章医生/KOL的看法
- 来自美国、欧盟和日本 4 位 KOL 的见解
第13章HER2低表现转移性乳癌的主要事件
- 扩大已核准的标靶治疗
- 订定新的可行目标
第 14 章 HER2 低表现转移性乳癌市场预测 - 2033 年
- 主要药物的市场预测与病患份额
第15章附录
The current clinical definition of HER2-low Breast Cancer (HER2-Low BC) used in clinical practice and ongoing clinical trials relies on the standard IHC and ISH approach; thus, tumors with low level of HER2 expression (defined as a HER2 IHC score of 1+ or 2+) and no detectable ERBB2 amplification fall into this category. It is a newly defined subset of HER2-negative BC that has HER2 immunohistochemical (IHC) score of 1+ or score of 2+/in situ hybridization (ISH) negative phenotype. IHC/ISH is the only standard technique currently applied to define HER2 expression.
"The treatment armamentarium for HER2-low metastatic breast cancer is rapidly evolving. Recent clinical trials have demonstrated the efficacy of CDK4/6 inhibitors in combination with endocrine therapy as a standard first-line treatment option. Additionally, the use of PI3K inhibitors and AKT inhibitors is being explored in clinical trials and may provide further treatment options in the near future."
HER2 low metastatic breast cancer is a new subtype of breast cancer which accounts for approximately 50%-60% of newly diagnosed breast cancer cases. This indicates that HER2 low breast cancer is a relatively common subtype of the disease. Even though HER2-low breast cancer has some HER2 expression, it is generally considered and treated as HER2 negative. Studies have shown that HER2-low expression is more common in HR+ breast cancer, but it can also be found in HR negative breast cancer (Won et al., 2021; Tan et al., 2021).
Mellalta Meets HER2-Low Expression in Breast Cancer: Evaluating the Evidence, Challenges, and Opportunities for Expanding Treatment Benefit to More Patients
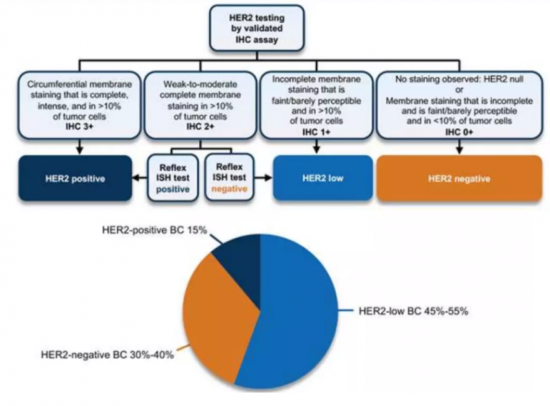
Mellalta's HER2-Low Metastatic Breast Cancer Deck: Current Treatment Landscape
Currently the mainstay of treatment for HER2-Low Metastatic Breast Cancer consist of combination of different therapeutic approaches like chemotherapy, endocrine therapies, targeted therapies.
In recent years, targeted therapies have shown promise in clinical trials and are being explored as alternative treatment options. The current standard of care for HER2-low metastatic breast cancer is rapidly evolving due to recent advancements in targeted therapies. Recent clinical trials have demonstrated significant clinical benefits of novel HER2-directed antibody-drug conjugates (ADCs) in treating HER2-low tumors. One such approved ADC is trastuzumab deruxtecan (T-Dxd), which has shown promising results in HER2-low breast cancer.
In addition to targeted therapies, endocrine therapy is also an important treatment option for HER2-low breast cancer, particularly in patients with hormone receptor-positive disease. Combination therapies, such as CDK4/6 inhibitors in combination with endocrine therapy, have also shown promise in improving outcomes for patients with HER2-low breast cancer.
"It is exciting that we have been able to now translate HER2-targeted therapy to a broader group of patients with HER2-low-expressing breast cancer. Overall, promising responses to T-DXd offer newfound treatment possibilities for a substantial number of patients, many of whom were previously considered to have limited therapeutic options. The recognition of HER2-low status also signals an opportunity to develop more precise, individualized therapeutic approaches through future research."
Mellalta's HER2-Low Metastatic Breast Cancer Deck: Current Unmet Needs
- Need for a clear and universally accepted definition of HER2-low, as the current classification is still evolving and there is ongoing research to determine the minimum threshold of HER2 expression required for treatment efficacy.
- HER2-low breast cancer (BC) has a poor prognosis, making the development of more suitable treatment an unmet clinical need.
- Need for standardized diagnostic criteria and guidelines for HER2-low tumors.
- Limited options for combination therapies that can enhance the efficacy of HER2-targeted treatments in HER2-low tumours.
"We are facing real challenges in terms of [HER2] identification in the clinic, and I would contend that we are in a state of flux in terms of the identification."
Mellalta's HER2-Low Metastatic Breast Cancer Deck: Key Takeaways
- Human epidermal growth factor receptor 2 (HER2) breast cancer, especially in the unresected, metastatic setting, is no longer considered solely binary as positive or negative.
- There is a new generation of approved antibody drug conjugate (ADC) like Trastuzumab deruxtecan (T-DXd) that have a higher drug-to-antibody ratio (DAR) and can deliver the toxin in a more effective manner for advanced unresectable, metastatic breast cancer.
- The 2023 National Comprehensive Cancer Network (NCCN) guidelines for the use of trastuzumab deruxtecan (T-DXd) reflect the clinical trial eligibility for DESTINY-Breast04. This allows health care professionals to use other ADCs approved for hormone receptor (HR)-positive and triple negative disease, irrespective of HER2 status.
- A traditional immunohistochemical (IHC) assay and scoring system have been used in testing to identify HER2-low tumors and the tumors with an IHC score of more than 0, less than 1+. New technologies may help to better identify patients with this subtype of tumor.
- The recently presented data of the DAISY trial suggested meaningful activity of T-DXd even in patients with HER2-0 metastatic breast cancer
Mellalta's HER2-Low Metastatic Breast Cancer Deck: Questions Answered:
- Potential challenges and opportunities in implementing targeted therapies for HER2-low breast cancer
- What is the size of clinically and commercially relevant drug-treatable HER2 low BC populations, and how will drug-treatment rates change over time?
- What is the expected market impact of recent drug approval such as Enhertu in treatment landscape of HER2-low metastatic BC?
- What are the most promising agents in the pipeline, and how will they shape the future of this therapy market?
- What key drivers and constraints will affect the HER2 low metastatic breast cancer therapy market over the forecast period?
Table of Content
1. Executive Summary
- 1.1. Summary of future trends
- 1.2. Potential opportunities to explore.
- 1.3. Drivers/barriers for entry
- 1.4. Unmet needs
- 1.5. What's new in HER2-Low Metastatic Breast Cancer?
2. HER2-Low Metastatic Breast Cancer Overview
- 2.1. HER2-Low Metastatic Breast Cancer definition, symptoms, etiology, Pathogenesis
- 2.2. Clinical Significance of HER2-Low Status
3. HER2-Low Metastatic Breast Cancer Definition & Diagnosis
- 3.1. Diagnostic Algorithm
- 3.2. HER2 Assessment with Immunohistochemistry (IHC) and In Situ Hybridization (ISH) (ASCO/CAP Guidelines)
- 3.3. AI-assisted interpretation of HER2 Status in HER2-Low Metastatic Breast Cancer
4. HER2-Low Metastatic Breast Cancer Epidemiology
- 4.1. Incidence rates by countries
5. HER2-Low Metastatic Breast Cancer Treatment Practices
- 5.1. Current treatment practices
- 5.2. Treatment algorithms
- 5.3. Acceptable endpoints for accelerated approval?
6. HER2-Low Metastatic Breast Cancer Approved Targeted Treatments
- 6.1. Quick overview of approved therapy
7. Pipeline clinical trials
- 7.1. HER2-Low Metastatic Breast Cancer pipeline landscape overview and analysis
- 7.2. Competitive Landscape of HER2-Low Metastatic Breast Cancer
- 7.3. Key molecules in clinical trials and results
- 7.4. Timeline of key drug approvals and launches.
8. Phase III Assets
- 8.1. Clinical trials and results
9. Phase II Assets
- 9.1. Clinical trials and results
10. Phase I Assets
- 10.1. Clinical trials and results
11. HER2-Low Metastatic Breast Cancer Pipeline Non-Clinical Molecules
- 11.1. Pre-clinical molecules
- 11.2. Mechanism of action, catalyst dates, and events
12. Physicians/KOLs Input
- 12.1. Insights from 4 KOLs in the US, EU, and Japan
13. Key Catalyst Events in HER2-Low Metastatic Breast Cancer
- 13.1. Expansion of approved targeted therapies
- 13.2. Creation of new actionable targets
14. HER2-Low Metastatic Breast Cancer Market Forecast -2033
- 14.1. Market Forecast and patient share by key drugs











