 |
市场调查报告书
商品编码
1298437
二尖瓣疾病市场:按治疗类型、按适应症、按最终用户:2022-2031 年全球机会分析和行业预测Mitral Valve Disease Market By Treatment Type, By Indication, By End User : Global Opportunity Analysis and Industry Forecast, 2022-2031 |
||||||
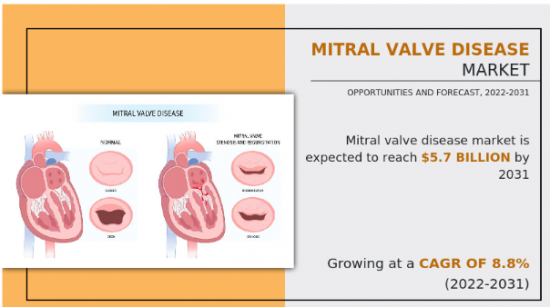
2021年二尖瓣疾病市场价值为244783万美元,预计到2031年将达到569253万美元,2022年至2031年復合年增长率为8.8%。
二尖瓣是心臟循环系统的重要组成部分,位于心臟左侧、左心房和左心室之间。它负责调节从左心房到左心室的血流。患病或受损的二尖瓣会导致各种问题,影响心臟有效泵血的能力。常见的二尖瓣病症包括二尖瓣狭窄、二尖瓣脱垂和二尖瓣关闭不全。
例如,瓣膜变窄会减少从左心房流入左心室的血量,导致左心房扩张,肺部压力增加。当这种情况持续存在时,就会出现呼吸急促、疲劳和胸痛等症状。在某些情况下,瓣膜无法正常关闭,导致血液回流到心房,从而引起类似的症状。无论哪种情况,治疗都包括使用药物和药物治疗症状,但严重的情况可能需要手术来修復或更换瓣膜。心臟再同步治疗可用于治疗二尖瓣关闭不全。
推动二尖瓣疾病市场增长的主要因素是二尖瓣疾病患病率的增加。被诊断患有二尖瓣疾病(包括二尖瓣关闭不全、二尖瓣脱垂和二尖瓣狭窄)的患者数量急剧增加。例如,根据《Journal of Clinical Medicine 2022》,二尖瓣关闭不全是欧洲第二常见的心臟瓣膜疾病,也是美国最常见的心臟瓣膜疾病,在 75 岁及以上人群中患病率为 9.3%。因此,二尖瓣疾病,如二尖瓣狭窄、二尖瓣脱垂、二尖瓣关闭不全等的患病率不断上升,催生了治疗药物、修復、置换手术、心臟同步治疗等多种治疗方法,对二尖瓣疾病的需求不断增加。选择,这反过来又有助于全球二尖瓣疾病市场的增长。
目录
第 1 章 简介
第二章执行摘要
第三章市场概况
- 市场定义和范围
- 主要发现
- 影响因素
- 主要投资口袋
- 波特五力分析
- 供应商的议价能力
- 买方议价能力
- 替代品的威胁
- 新进入者的威胁
- 竞争强度
- 市场动态
- 促进者
- 二尖瓣疾病患病率增加
- 对微创治疗的需求不断增长
- 老年人口增加
- 越来越多的产品批准用于治疗二尖瓣疾病
- 阻碍因素
- 二尖瓣手术的高成本和相关风险
- 严格监管:管理机构
- 机会
- 技术进步和新型二尖瓣的推出
- 医疗支出增加
- 促进者
- 分析 COVID-19 对市场的影响
4.二尖瓣疾病市场,按治疗类型
- 概述
- 市场规模和预测
- 二尖瓣修復术
- 主要市场趋势、增长动力和机遇
- 市场规模/预测:按地区
- 市场份额分析:按国家分类
- 二尖瓣置换术
- 主要市场趋势、增长动力和机遇
- 市场规模/预测:按地区
- 市场份额分析:按国家分类
- 二尖瓣置换术 二尖瓣疾病市场,按产品类型
- 心臟再同步治疗
- 主要市场趋势、增长动力和机遇
- 市场规模/预测:按地区
- 市场份额分析:按国家分类
- 二尖瓣治疗
- 主要市场趋势、增长动力和机遇
- 市场规模/预测:按地区
- 市场份额分析:按国家分类
- 二尖瓣治疗的二尖瓣疾病市场:按药物类别
5. 二尖瓣疾病市场(按适应症)
- 概述
- 市场规模及预测
- 二尖瓣狭窄
- 主要市场趋势、增长动力和机遇
- 市场规模/预测:按地区
- 市场份额分析:按国家分类
- 二尖瓣脱垂
- 主要市场趋势、增长动力和机遇
- 市场规模/预测:按地区
- 市场份额分析:按国家分类
- 二尖瓣关闭不全
- 主要市场趋势、增长动力和机遇
- 市场规模/预测:按地区
- 市场份额分析:按国家分类
- 二尖瓣反流二尖瓣疾病市场,按类型
6. 二尖瓣疾病市场(按最终用户)
- 概述
- 市场规模及预测
- 医院
- 主要市场趋势、增长动力和机遇
- 市场规模/预测:按地区
- 市场份额分析:按国家分类
- 门诊手术中心
- 主要市场趋势、增长动力和机遇
- 市场规模/预测:按地区
- 市场份额分析:按国家分类
- 其他
- 主要市场趋势、增长动力和机遇
- 市场规模/预测:按地区
- 市场份额分析:按国家分类
7.二尖瓣疾病市场,按地区
- 概述
- 市场规模/预测:按地区
- 北美
- 主要趋势和机遇
- 市场规模和预测:按治疗类型
- 市场规模/预测:按迹象显示
- 市场规模/预测:按最终用户分类
- 市场规模/预测:按国家
- 美国
- 主要市场趋势、增长动力和机遇
- 市场规模和预测:按治疗类型
- 市场规模/预测:按迹象显示
- 市场规模/预测:按最终用户分类
- 加拿大
- 主要市场趋势、增长动力和机遇
- 市场规模和预测:按治疗类型
- 市场规模/预测:按迹象显示
- 市场规模/预测:按最终用户分类
- 墨西哥
- 主要市场趋势、增长动力和机遇
- 市场规模和预测:按治疗类型
- 市场规模/预测:按迹象显示
- 市场规模/预测:按最终用户分类
- 欧洲
- 主要趋势和机遇
- 市场规模和预测:按治疗类型
- 市场规模/预测:按迹象显示
- 市场规模/预测:按最终用户分类
- 市场规模/预测:按国家
- 德国
- 主要市场趋势、增长动力和机遇
- 市场规模和预测:按治疗类型
- 市场规模/预测:按迹象显示
- 市场规模/预测:按最终用户分类
- 法国
- 主要市场趋势、增长动力和机遇
- 市场规模和预测:按治疗类型
- 市场规模/预测:按迹象显示
- 市场规模/预测:按最终用户分类
- 英国
- 主要市场趋势、增长动力和机遇
- 市场规模和预测:按治疗类型
- 市场规模/预测:按迹象显示
- 市场规模/预测:按最终用户分类
- 意大利
- 主要市场趋势、增长动力和机遇
- 市场规模和预测:按治疗类型
- 市场规模/预测:按迹象显示
- 市场规模/预测:按最终用户分类
- 西班牙
- 主要市场趋势、增长动力和机遇
- 市场规模和预测:按治疗类型
- 市场规模/预测:按迹象显示
- 市场规模/预测:按最终用户分类
- 欧洲其他地区
- 主要市场趋势、增长动力和机遇
- 市场规模和预测:按治疗类型
- 市场规模/预测:按迹象显示
- 市场规模/预测:按最终用户分类
- 亚太地区
- 主要趋势和机遇
- 市场规模和预测:按治疗类型
- 市场规模/预测:按迹象显示
- 市场规模/预测:按最终用户分类
- 市场规模/预测:按国家
- 日本
- 主要市场趋势、增长动力和机遇
- 市场规模和预测:按治疗类型
- 市场规模/预测:按迹象显示
- 市场规模/预测:按最终用户分类
- 中国
- 主要市场趋势、增长动力和机遇
- 市场规模和预测:按治疗类型
- 市场规模/预测:按迹象显示
- 市场规模/预测:按最终用户分类
- 印度
- 主要市场趋势、增长动力和机遇
- 市场规模和预测:按治疗类型
- 市场规模/预测:按迹象显示
- 市场规模/预测:按最终用户分类
- 澳大利亚
- 主要市场趋势、增长动力和机遇
- 市场规模和预测:按治疗类型
- 市场规模/预测:按迹象显示
- 市场规模/预测:按最终用户分类
- 韩国
- 主要市场趋势、增长动力和机遇
- 市场规模和预测:按治疗类型
- 市场规模/预测:按迹象显示
- 市场规模/预测:按最终用户分类
- 亚太其他地区
- 主要市场趋势、增长动力和机遇
- 市场规模和预测:按治疗类型
- 市场规模/预测:按迹象显示
- 市场规模/预测:按最终用户分类
- 拉丁美洲/中东/非洲
- 主要趋势和机遇
- 市场规模和预测:按治疗类型
- 市场规模/预测:按迹象显示
- 市场规模/预测:按最终用户分类
- 市场规模/预测:按国家
- 巴西
- 主要市场趋势、增长动力和机遇
- 市场规模和预测:按治疗类型
- 市场规模/预测:按迹象显示
- 市场规模/预测:按最终用户分类
- 沙特阿拉伯
- 主要市场趋势、增长动力和机遇
- 市场规模和预测:按治疗类型
- 市场规模/预测:按迹象显示
- 市场规模/预测:按最终用户分类
- 南非
- 主要市场趋势、增长动力和机遇
- 市场规模和预测:按治疗类型
- 市场规模/预测:按迹象显示
- 市场规模/预测:按最终用户分类
- 其他地区
- 主要市场趋势、增长动力和机遇
- 市场规模和预测:按治疗类型
- 市场规模/预测:按迹象显示
- 市场规模/预测:按最终用户分类
第8章 竞争格局
- 介绍
- 关键成功策略
- 10大公司产品图
- 比赛仪表板
- 比赛热图
- 2021 年顶级公司定位
第九章公司简介
- Abbott Laboratories
- Affluent Medical
- Corcym UK Limited
- Edwards Lifesciences Corporation
- Labcor Laboratorios Ltda
- Medtronic plc
- ShockWave Medical, Inc.
- Valcare Medical
- Artivion, Inc.
- Braile Biomedica
- Zydus Lifesciences Limited
- Teva Pharmaceutical Industries Ltd.
- Pfizer Inc.
- Bayer AG
- Novartis AG
The Mitral Valve Disease Market valued for $2,447.83 million in 2021 and is estimated to reach $5,692.53 million by 2031, exhibiting a CAGR of 8.8% from 2022 to 2031.
Mitral valve is an essential component of the heart's circulatory system that is located on the left side of the heart between the left atrium and left ventricle. It is responsible for regulating the flow of blood from the left atrium into the left ventricle. When the mitral valve is diseased or damaged, it can lead to a range of problems that can affect the heart's ability to pump blood efficiently. Common mitral valve conditions include mitral valve stenosis, mitral valve prolapse, and mitral valve regurgitation.
For example, if the valve is narrowed, the amount of blood that can flow from the left atrium into the left ventricle is reduced, which can cause the left atrium to enlarge, thus increasing the pressure in lungs. These conditions can lead to symptoms such as shortness of breath, fatigue, and chest pain. In some cases, the valve is not able to close properly, causing the blood to flow back into the atrium, which can lead to similar symptoms. In both cases, treatment may involve medication or therapeutics to manage symptoms, but in severe cases, surgery may be required to repair or replace the valve. In some cases of mitral valve regurgitation, cardiac resynchronization therapy may be used to treat the condition.

The major factor driving the growth of mitral valve disease market is increase in prevalence of mitral valve diseases. Alarming increase has been witnessed in the number of patients diagnosed with mitral valve diseases such as mitral regurgitation, mitral prolapse, and mitral stenosis. For instance, Journal of Clinical Medicine 2022 stated that mitral regurgitation is the second most common valvular heart disease in Europe and the most common in the U.S., with a prevalence of 9.3% in the population aged over 75 years. Thus, rise in prevalence of mitral valve diseases such as mitral stenosis, mitral prolapse, and mitral regurgitation escalates the demand for various treatment options, including therapeutics, repair, and replacement surgeries and cardiac synchronization therapy, which, in turn, contributes toward the growth of the global mitral valve disease market.
In addition, technological advancements have played a significant role in driving the market growth in recent years. As the prevalence of mitral valve diseases rises, the demand for better treatment options that can provide improved outcomes and reduced recovery times increases simultaneously. One of the most significant technological advancements in this field is the development of minimally invasive techniques such as transcatheter mitral valve replacement (TMVR). This technique involves replacing the diseased valve with an artificial valve through a small incision in the groin or chest. This offers several advantages over traditional open-heart surgery, such as reduced recovery time and lower risk of complications. Thus, huge demand for technologically advanced mitral valve treatment devices in the healthcare sector fuels the growth of the market.
Furthermore, surge in geriatric population drives the demand for mitral valve disease treatments around the globe, owing to the fact that aged individuals are highly susceptible to mitral valve diseases. Moreover, with growing age , the valve can be damaged or weakened, making it more prone to malfunction. In addition, rise in risk factors such as high blood pressure and obesity can increase the risk of developing mitral valve disease, which is anticipated to foster the growth of mitral valve disease market.
However, the cost of mitral valve treatment procedures such as repair, replacement, and cardiac synchronization therapy is significantly high, which acts as a barrier for the market growth. In addition, low awareness among the population in underdeveloped countries regarding the available treatments hinders the market growth. Moreover, stringent regulations by the governing bodies for the development and launching of mitral valve treatment products may indeed be a hindrance to market growth.
On the contrary, increase in demand for advanced healthcare services, rise in awareness of mitral valve treatment medications & procedures, and government investments in healthcare infrastructure are expected to provide remunerative opportunities for the expansion of the market during the forecast period. Governments in Asia-Pacific and LAMEA are substantially investing in the development of healthcare infrastructure and increasing the access to healthcare services, which notably drive the growth of the mitral valve disease market.
The global mitral valve disease market is segmented into treatment type, indication, end user, and region. On the basis of treatment type, the market is fragmented into mitral valve repair, mitral valve replacement, cardiac resynchronization therapy (CRT), and mitral valve therapeutics. The mitral valve replacement segment is further bifurcated into mechanical valves and biological valves (bioprosthetic tissue). On the other hand, the mitral valve therapeutics segment is subdivided into beta blockers, diuretics, anticoagulants, and others.
Depending on indication, the market is fragmented into mitral valve stenosis, mitral valve prolapse, and mitral valve regurgitation. Furthermore, mitral valve regurgitation segment is bifurcated into primary (degenerative) mitral valve regurgitation and secondary (functional) mitral valve regurgitation. By end user, the market is segregated into hospitals, ambulatory surgical centers, and others.
Region wise, the market is analyzed across North America (the U.S., Canada, and Mexico), Europe (Germany, France, the UK, Italy, Spain, and rest of Europe), Asia-Pacific (Japan, China, Australia, India, South Korea, and rest of Asia-Pacific), and LAMEA (Brazil, South Africa, Saudi Arabia, and rest of LAMEA).
The major players that operate in the mitral valve disease market include: Valcare Medical, Affluent Medical, Abbott Laboratories, Corcym UK Limited, Edwards Lifesciences Corporation, Medtronic Plc., ShockWave Medical, Inc., Artivion, Inc., Labcor Laboratorios Ltda, Braile Biomedica, Zydus Lifesciences Limited, Teva Pharmaceutical Industries Ltd., Pfizer Inc., Bayer AG, and Novartis AG.
Key Benefits For Stakeholders
- This report provides a quantitative analysis of the market segments, current trends, estimations, and dynamics of the mitral valve disease market analysis from 2021 to 2031 to identify the prevailing mitral valve disease market opportunities.
- The market research is offered along with information related to key drivers, restraints, and opportunities.
- Porter's five forces analysis highlights the potency of buyers and suppliers to enable stakeholders make profit-oriented business decisions and strengthen their supplier-buyer network.
- In-depth analysis of the mitral valve disease market segmentation assists to determine the prevailing market opportunities.
- Major countries in each region are mapped according to their revenue contribution to the global market.
- Market player positioning facilitates benchmarking and provides a clear understanding of the present position of the market players.
- The report includes the analysis of the regional as well as global mitral valve disease market trends, key players, market segments, application areas, and market growth strategies.
Key Market Segments
By Treatment Type
- Mitral Valve Repair
- Mitral Valve Replacement
- Product Type
- Mechanical Valves
- Biological Valves
- Cardiac Resynchronization Therapy
- Mitral Valve Therapeutics
- Drug Class
- Beta blockers
- Diuretics
- Anticoagulants
- Others
By Indication
- Mitral Valve Stenosis
- Mitral Valve Prolapse
- Mitral Valve Regurgitation
- Type
- Primary Mitral Valve Regurgitation
- Secondary Mitral Valve Regurgitation
By End User
- Hospitals
- Ambulatory Surgical Centers
- Others
By Region
- North America
- U.S.
- Canada
- Mexico
- Europe
- Germany
- France
- UK
- Italy
- Spain
- Rest of Europe
- Asia-Pacific
- Japan
- China
- India
- Australia
- South Korea
- Rest of Asia-Pacific
- LAMEA
- Brazil
- Saudi Arabia
- South Africa
- Rest of LAMEA
Key Market Players:
- Bayer AG
- Zydus Lifesciences Limited
- Affluent Medical
- Artivion, Inc.
- Valcare Medical
- Corcym UK Limited
- Pfizer Inc.
- Novartis AG
- Teva Pharmaceutical Industries Ltd.
- Medtronic plc
- ShockWave Medical, Inc.
- Braile Biomedica
- Abbott Laboratories
- Labcor Laboratorios Ltda
- Edwards Lifesciences Corporation
TABLE OF CONTENTS
CHAPTER 1: INTRODUCTION
- 1.1. Report description
- 1.2. Key market segments
- 1.3. Key benefits to the stakeholders
- 1.4. Research Methodology
- 1.4.1. Primary research
- 1.4.2. Secondary research
- 1.4.3. Analyst tools and models
CHAPTER 2: EXECUTIVE SUMMARY
- 2.1. CXO Perspective
CHAPTER 3: MARKET OVERVIEW
- 3.1. Market definition and scope
- 3.2. Key findings
- 3.2.1. Top impacting factors
- 3.2.2. Top investment pockets
- 3.3. Porter's five forces analysis
- 3.3.1. Bargaining power of suppliers
- 3.3.2. Bargaining power of buyers
- 3.3.3. Threat of substitutes
- 3.3.4. Threat of new entrants
- 3.3.5. Intensity of rivalry
- 3.4. Market dynamics
- 3.4.1. Drivers
- 3.4.1.1. Increase in prevalence of mitral valve diseases
- 3.4.1.2. Increase in demand for minimally invasive procedures
- 3.4.1.3. Rise in geriatric population
- 3.4.1.4. Increase in product approvals for mitral valve diseases treatment
- 3.4.1. Drivers
- 3.4.2. Restraints
- 3.4.2.1. High cost of mitral valve surgeries and risks associated with the procedure
- 3.4.2.2. Stringent regulations by the governing bodies
- 3.4.3. Opportunities
- 3.4.3.1. Technological advancements and introduction of novel mitral valves
- 3.4.3.2. Rise in healthcare expenditure
- 3.5. COVID-19 Impact Analysis on the market
CHAPTER 4: MITRAL VALVE DISEASE MARKET, BY TREATMENT TYPE
- 4.1. Overview
- 4.1.1. Market size and forecast
- 4.2. Mitral Valve Repair
- 4.2.1. Key market trends, growth factors and opportunities
- 4.2.2. Market size and forecast, by region
- 4.2.3. Market share analysis by country
- 4.3. Mitral Valve Replacement
- 4.3.1. Key market trends, growth factors and opportunities
- 4.3.2. Market size and forecast, by region
- 4.3.3. Market share analysis by country
- 4.3.4. Mitral Valve Replacement Mitral Valve Disease Market by Product Type
- 4.4. Cardiac Resynchronization Therapy
- 4.4.1. Key market trends, growth factors and opportunities
- 4.4.2. Market size and forecast, by region
- 4.4.3. Market share analysis by country
- 4.5. Mitral Valve Therapeutics
- 4.5.1. Key market trends, growth factors and opportunities
- 4.5.2. Market size and forecast, by region
- 4.5.3. Market share analysis by country
- 4.5.4. Mitral Valve Therapeutics Mitral Valve Disease Market by Drug Class
CHAPTER 5: MITRAL VALVE DISEASE MARKET, BY INDICATION
- 5.1. Overview
- 5.1.1. Market size and forecast
- 5.2. Mitral Valve Stenosis
- 5.2.1. Key market trends, growth factors and opportunities
- 5.2.2. Market size and forecast, by region
- 5.2.3. Market share analysis by country
- 5.3. Mitral Valve Prolapse
- 5.3.1. Key market trends, growth factors and opportunities
- 5.3.2. Market size and forecast, by region
- 5.3.3. Market share analysis by country
- 5.4. Mitral Valve Regurgitation
- 5.4.1. Key market trends, growth factors and opportunities
- 5.4.2. Market size and forecast, by region
- 5.4.3. Market share analysis by country
- 5.4.4. Mitral Valve Regurgitation Mitral Valve Disease Market by Type
CHAPTER 6: MITRAL VALVE DISEASE MARKET, BY END USER
- 6.1. Overview
- 6.1.1. Market size and forecast
- 6.2. Hospitals
- 6.2.1. Key market trends, growth factors and opportunities
- 6.2.2. Market size and forecast, by region
- 6.2.3. Market share analysis by country
- 6.3. Ambulatory Surgical Centers
- 6.3.1. Key market trends, growth factors and opportunities
- 6.3.2. Market size and forecast, by region
- 6.3.3. Market share analysis by country
- 6.4. Others
- 6.4.1. Key market trends, growth factors and opportunities
- 6.4.2. Market size and forecast, by region
- 6.4.3. Market share analysis by country
CHAPTER 7: MITRAL VALVE DISEASE MARKET, BY REGION
- 7.1. Overview
- 7.1.1. Market size and forecast By Region
- 7.2. North America
- 7.2.1. Key trends and opportunities
- 7.2.2. Market size and forecast, by Treatment Type
- 7.2.3. Market size and forecast, by Indication
- 7.2.4. Market size and forecast, by End User
- 7.2.5. Market size and forecast, by country
- 7.2.5.1. U.S.
- 7.2.5.1.1. Key market trends, growth factors and opportunities
- 7.2.5.1.2. Market size and forecast, by Treatment Type
- 7.2.5.1.3. Market size and forecast, by Indication
- 7.2.5.1.4. Market size and forecast, by End User
- 7.2.5.2. Canada
- 7.2.5.2.1. Key market trends, growth factors and opportunities
- 7.2.5.2.2. Market size and forecast, by Treatment Type
- 7.2.5.2.3. Market size and forecast, by Indication
- 7.2.5.2.4. Market size and forecast, by End User
- 7.2.5.3. Mexico
- 7.2.5.3.1. Key market trends, growth factors and opportunities
- 7.2.5.3.2. Market size and forecast, by Treatment Type
- 7.2.5.3.3. Market size and forecast, by Indication
- 7.2.5.3.4. Market size and forecast, by End User
- 7.3. Europe
- 7.3.1. Key trends and opportunities
- 7.3.2. Market size and forecast, by Treatment Type
- 7.3.3. Market size and forecast, by Indication
- 7.3.4. Market size and forecast, by End User
- 7.3.5. Market size and forecast, by country
- 7.3.5.1. Germany
- 7.3.5.1.1. Key market trends, growth factors and opportunities
- 7.3.5.1.2. Market size and forecast, by Treatment Type
- 7.3.5.1.3. Market size and forecast, by Indication
- 7.3.5.1.4. Market size and forecast, by End User
- 7.3.5.2. France
- 7.3.5.2.1. Key market trends, growth factors and opportunities
- 7.3.5.2.2. Market size and forecast, by Treatment Type
- 7.3.5.2.3. Market size and forecast, by Indication
- 7.3.5.2.4. Market size and forecast, by End User
- 7.3.5.3. UK
- 7.3.5.3.1. Key market trends, growth factors and opportunities
- 7.3.5.3.2. Market size and forecast, by Treatment Type
- 7.3.5.3.3. Market size and forecast, by Indication
- 7.3.5.3.4. Market size and forecast, by End User
- 7.3.5.4. Italy
- 7.3.5.4.1. Key market trends, growth factors and opportunities
- 7.3.5.4.2. Market size and forecast, by Treatment Type
- 7.3.5.4.3. Market size and forecast, by Indication
- 7.3.5.4.4. Market size and forecast, by End User
- 7.3.5.5. Spain
- 7.3.5.5.1. Key market trends, growth factors and opportunities
- 7.3.5.5.2. Market size and forecast, by Treatment Type
- 7.3.5.5.3. Market size and forecast, by Indication
- 7.3.5.5.4. Market size and forecast, by End User
- 7.3.5.6. Rest of Europe
- 7.3.5.6.1. Key market trends, growth factors and opportunities
- 7.3.5.6.2. Market size and forecast, by Treatment Type
- 7.3.5.6.3. Market size and forecast, by Indication
- 7.3.5.6.4. Market size and forecast, by End User
- 7.4. Asia-Pacific
- 7.4.1. Key trends and opportunities
- 7.4.2. Market size and forecast, by Treatment Type
- 7.4.3. Market size and forecast, by Indication
- 7.4.4. Market size and forecast, by End User
- 7.4.5. Market size and forecast, by country
- 7.4.5.1. Japan
- 7.4.5.1.1. Key market trends, growth factors and opportunities
- 7.4.5.1.2. Market size and forecast, by Treatment Type
- 7.4.5.1.3. Market size and forecast, by Indication
- 7.4.5.1.4. Market size and forecast, by End User
- 7.4.5.2. China
- 7.4.5.2.1. Key market trends, growth factors and opportunities
- 7.4.5.2.2. Market size and forecast, by Treatment Type
- 7.4.5.2.3. Market size and forecast, by Indication
- 7.4.5.2.4. Market size and forecast, by End User
- 7.4.5.3. India
- 7.4.5.3.1. Key market trends, growth factors and opportunities
- 7.4.5.3.2. Market size and forecast, by Treatment Type
- 7.4.5.3.3. Market size and forecast, by Indication
- 7.4.5.3.4. Market size and forecast, by End User
- 7.4.5.4. Australia
- 7.4.5.4.1. Key market trends, growth factors and opportunities
- 7.4.5.4.2. Market size and forecast, by Treatment Type
- 7.4.5.4.3. Market size and forecast, by Indication
- 7.4.5.4.4. Market size and forecast, by End User
- 7.4.5.5. South Korea
- 7.4.5.5.1. Key market trends, growth factors and opportunities
- 7.4.5.5.2. Market size and forecast, by Treatment Type
- 7.4.5.5.3. Market size and forecast, by Indication
- 7.4.5.5.4. Market size and forecast, by End User
- 7.4.5.6. Rest of Asia-Pacific
- 7.4.5.6.1. Key market trends, growth factors and opportunities
- 7.4.5.6.2. Market size and forecast, by Treatment Type
- 7.4.5.6.3. Market size and forecast, by Indication
- 7.4.5.6.4. Market size and forecast, by End User
- 7.5. LAMEA
- 7.5.1. Key trends and opportunities
- 7.5.2. Market size and forecast, by Treatment Type
- 7.5.3. Market size and forecast, by Indication
- 7.5.4. Market size and forecast, by End User
- 7.5.5. Market size and forecast, by country
- 7.5.5.1. Brazil
- 7.5.5.1.1. Key market trends, growth factors and opportunities
- 7.5.5.1.2. Market size and forecast, by Treatment Type
- 7.5.5.1.3. Market size and forecast, by Indication
- 7.5.5.1.4. Market size and forecast, by End User
- 7.5.5.2. Saudi Arabia
- 7.5.5.2.1. Key market trends, growth factors and opportunities
- 7.5.5.2.2. Market size and forecast, by Treatment Type
- 7.5.5.2.3. Market size and forecast, by Indication
- 7.5.5.2.4. Market size and forecast, by End User
- 7.5.5.3. South Africa
- 7.5.5.3.1. Key market trends, growth factors and opportunities
- 7.5.5.3.2. Market size and forecast, by Treatment Type
- 7.5.5.3.3. Market size and forecast, by Indication
- 7.5.5.3.4. Market size and forecast, by End User
- 7.5.5.4. Rest of LAMEA
- 7.5.5.4.1. Key market trends, growth factors and opportunities
- 7.5.5.4.2. Market size and forecast, by Treatment Type
- 7.5.5.4.3. Market size and forecast, by Indication
- 7.5.5.4.4. Market size and forecast, by End User
CHAPTER 8: COMPETITIVE LANDSCAPE
- 8.1. Introduction
- 8.2. Top winning strategies
- 8.3. Product Mapping of Top 10 Player
- 8.4. Competitive Dashboard
- 8.5. Competitive Heatmap
- 8.6. Top player positioning, 2021
CHAPTER 9: COMPANY PROFILES
- 9.1. Abbott Laboratories
- 9.1.1. Company overview
- 9.1.2. Key Executives
- 9.1.3. Company snapshot
- 9.1.4. Operating business segments
- 9.1.5. Product portfolio
- 9.1.6. Business performance
- 9.1.7. Key strategic moves and developments
- 9.2. Affluent Medical
- 9.2.1. Company overview
- 9.2.2. Key Executives
- 9.2.3. Company snapshot
- 9.2.4. Operating business segments
- 9.2.5. Product portfolio
- 9.2.6. Key strategic moves and developments
- 9.3. Corcym UK Limited
- 9.3.1. Company overview
- 9.3.2. Key Executives
- 9.3.3. Company snapshot
- 9.3.4. Operating business segments
- 9.3.5. Product portfolio
- 9.3.6. Key strategic moves and developments
- 9.4. Edwards Lifesciences Corporation
- 9.4.1. Company overview
- 9.4.2. Key Executives
- 9.4.3. Company snapshot
- 9.4.4. Operating business segments
- 9.4.5. Product portfolio
- 9.4.6. Business performance
- 9.4.7. Key strategic moves and developments
- 9.5. Labcor Laboratorios Ltda
- 9.5.1. Company overview
- 9.5.2. Key Executives
- 9.5.3. Company snapshot
- 9.5.4. Operating business segments
- 9.5.5. Product portfolio
- 9.6. Medtronic plc
- 9.6.1. Company overview
- 9.6.2. Key Executives
- 9.6.3. Company snapshot
- 9.6.4. Operating business segments
- 9.6.5. Product portfolio
- 9.6.6. Business performance
- 9.6.7. Key strategic moves and developments
- 9.7. ShockWave Medical, Inc.
- 9.7.1. Company overview
- 9.7.2. Key Executives
- 9.7.3. Company snapshot
- 9.7.4. Operating business segments
- 9.7.5. Product portfolio
- 9.7.6. Business performance
- 9.7.7. Key strategic moves and developments
- 9.8. Valcare Medical
- 9.8.1. Company overview
- 9.8.2. Key Executives
- 9.8.3. Company snapshot
- 9.8.4. Operating business segments
- 9.8.5. Product portfolio
- 9.9. Artivion, Inc.
- 9.9.1. Company overview
- 9.9.2. Key Executives
- 9.9.3. Company snapshot
- 9.9.4. Operating business segments
- 9.9.5. Product portfolio
- 9.9.6. Business performance
- 9.9.7. Key strategic moves and developments
- 9.10. Braile Biomedica
- 9.10.1. Company overview
- 9.10.2. Key Executives
- 9.10.3. Company snapshot
- 9.10.4. Operating business segments
- 9.10.5. Product portfolio
- 9.11. Zydus Lifesciences Limited
- 9.11.1. Company overview
- 9.11.2. Key Executives
- 9.11.3. Company snapshot
- 9.11.4. Operating business segments
- 9.11.5. Product portfolio
- 9.11.6. Business performance
- 9.12. Teva Pharmaceutical Industries Ltd.
- 9.12.1. Company overview
- 9.12.2. Key Executives
- 9.12.3. Company snapshot
- 9.12.4. Operating business segments
- 9.12.5. Product portfolio
- 9.12.6. Business performance
- 9.13. Pfizer Inc.
- 9.13.1. Company overview
- 9.13.2. Key Executives
- 9.13.3. Company snapshot
- 9.13.4. Operating business segments
- 9.13.5. Product portfolio
- 9.13.6. Business performance
- 9.14. Bayer AG
- 9.14.1. Company overview
- 9.14.2. Key Executives
- 9.14.3. Company snapshot
- 9.14.4. Operating business segments
- 9.14.5. Product portfolio
- 9.14.6. Business performance
- 9.15. Novartis AG
- 9.15.1. Company overview
- 9.15.2. Key Executives
- 9.15.3. Company snapshot
- 9.15.4. Operating business segments
- 9.15.5. Product portfolio
- 9.15.6. Business performance
LIST OF TABLES
- TABLE 01. GLOBAL MITRAL VALVE DISEASE MARKET, BY TREATMENT TYPE, 2021-2031 ($MILLION)
- TABLE 02. MITRAL VALVE DISEASE MARKET FOR MITRAL VALVE REPAIR, BY REGION, 2021-2031 ($MILLION)
- TABLE 03. MITRAL VALVE DISEASE MARKET FOR MITRAL VALVE REPLACEMENT, BY REGION, 2021-2031 ($MILLION)
- TABLE 04. GLOBAL MITRAL VALVE REPLACEMENT MITRAL VALVE DISEASE MARKET, BY PRODUCT TYPE, 2021-2031 ($MILLION)
- TABLE 05. MITRAL VALVE DISEASE MARKET FOR CARDIAC RESYNCHRONIZATION THERAPY, BY REGION, 2021-2031 ($MILLION)
- TABLE 06. MITRAL VALVE DISEASE MARKET FOR MITRAL VALVE THERAPEUTICS, BY REGION, 2021-2031 ($MILLION)
- TABLE 07. GLOBAL MITRAL VALVE THERAPEUTICS MITRAL VALVE DISEASE MARKET, BY DRUG CLASS, 2021-2031 ($MILLION)
- TABLE 08. GLOBAL MITRAL VALVE DISEASE MARKET, BY INDICATION, 2021-2031 ($MILLION)
- TABLE 09. MITRAL VALVE DISEASE MARKET FOR MITRAL VALVE STENOSIS, BY REGION, 2021-2031 ($MILLION)
- TABLE 10. MITRAL VALVE DISEASE MARKET FOR MITRAL VALVE PROLAPSE, BY REGION, 2021-2031 ($MILLION)
- TABLE 11. MITRAL VALVE DISEASE MARKET FOR MITRAL VALVE REGURGITATION, BY REGION, 2021-2031 ($MILLION)
- TABLE 12. GLOBAL MITRAL VALVE REGURGITATION MITRAL VALVE DISEASE MARKET, BY TYPE, 2021-2031 ($MILLION)
- TABLE 13. GLOBAL MITRAL VALVE DISEASE MARKET, BY END USER, 2021-2031 ($MILLION)
- TABLE 14. MITRAL VALVE DISEASE MARKET FOR HOSPITALS, BY REGION, 2021-2031 ($MILLION)
- TABLE 15. MITRAL VALVE DISEASE MARKET FOR AMBULATORY SURGICAL CENTERS, BY REGION, 2021-2031 ($MILLION)
- TABLE 16. MITRAL VALVE DISEASE MARKET FOR OTHERS, BY REGION, 2021-2031 ($MILLION)
- TABLE 17. MITRAL VALVE DISEASE MARKET, BY REGION, 2021-2031 ($MILLION)
- TABLE 18. NORTH AMERICA MITRAL VALVE DISEASE MARKET, BY TREATMENT TYPE, 2021-2031 ($MILLION)
- TABLE 19. NORTH AMERICA MITRAL VALVE DISEASE MARKET, BY INDICATION, 2021-2031 ($MILLION)
- TABLE 20. NORTH AMERICA MITRAL VALVE DISEASE MARKET, BY END USER, 2021-2031 ($MILLION)
- TABLE 21. NORTH AMERICA MITRAL VALVE DISEASE MARKET, BY COUNTRY, 2021-2031 ($MILLION)
- TABLE 22. U.S. MITRAL VALVE DISEASE MARKET, BY TREATMENT TYPE, 2021-2031 ($MILLION)
- TABLE 23. U.S. MITRAL VALVE DISEASE MARKET, BY INDICATION, 2021-2031 ($MILLION)
- TABLE 24. U.S. MITRAL VALVE DISEASE MARKET, BY END USER, 2021-2031 ($MILLION)
- TABLE 25. CANADA MITRAL VALVE DISEASE MARKET, BY TREATMENT TYPE, 2021-2031 ($MILLION)
- TABLE 26. CANADA MITRAL VALVE DISEASE MARKET, BY INDICATION, 2021-2031 ($MILLION)
- TABLE 27. CANADA MITRAL VALVE DISEASE MARKET, BY END USER, 2021-2031 ($MILLION)
- TABLE 28. MEXICO MITRAL VALVE DISEASE MARKET, BY TREATMENT TYPE, 2021-2031 ($MILLION)
- TABLE 29. MEXICO MITRAL VALVE DISEASE MARKET, BY INDICATION, 2021-2031 ($MILLION)
- TABLE 30. MEXICO MITRAL VALVE DISEASE MARKET, BY END USER, 2021-2031 ($MILLION)
- TABLE 31. EUROPE MITRAL VALVE DISEASE MARKET, BY TREATMENT TYPE, 2021-2031 ($MILLION)
- TABLE 32. EUROPE MITRAL VALVE DISEASE MARKET, BY INDICATION, 2021-2031 ($MILLION)
- TABLE 33. EUROPE MITRAL VALVE DISEASE MARKET, BY END USER, 2021-2031 ($MILLION)
- TABLE 34. EUROPE MITRAL VALVE DISEASE MARKET, BY COUNTRY, 2021-2031 ($MILLION)
- TABLE 35. GERMANY MITRAL VALVE DISEASE MARKET, BY TREATMENT TYPE, 2021-2031 ($MILLION)
- TABLE 36. GERMANY MITRAL VALVE DISEASE MARKET, BY INDICATION, 2021-2031 ($MILLION)
- TABLE 37. GERMANY MITRAL VALVE DISEASE MARKET, BY END USER, 2021-2031 ($MILLION)
- TABLE 38. FRANCE MITRAL VALVE DISEASE MARKET, BY TREATMENT TYPE, 2021-2031 ($MILLION)
- TABLE 39. FRANCE MITRAL VALVE DISEASE MARKET, BY INDICATION, 2021-2031 ($MILLION)
- TABLE 40. FRANCE MITRAL VALVE DISEASE MARKET, BY END USER, 2021-2031 ($MILLION)
- TABLE 41. UK MITRAL VALVE DISEASE MARKET, BY TREATMENT TYPE, 2021-2031 ($MILLION)
- TABLE 42. UK MITRAL VALVE DISEASE MARKET, BY INDICATION, 2021-2031 ($MILLION)
- TABLE 43. UK MITRAL VALVE DISEASE MARKET, BY END USER, 2021-2031 ($MILLION)
- TABLE 44. ITALY MITRAL VALVE DISEASE MARKET, BY TREATMENT TYPE, 2021-2031 ($MILLION)
- TABLE 45. ITALY MITRAL VALVE DISEASE MARKET, BY INDICATION, 2021-2031 ($MILLION)
- TABLE 46. ITALY MITRAL VALVE DISEASE MARKET, BY END USER, 2021-2031 ($MILLION)
- TABLE 47. SPAIN MITRAL VALVE DISEASE MARKET, BY TREATMENT TYPE, 2021-2031 ($MILLION)
- TABLE 48. SPAIN MITRAL VALVE DISEASE MARKET, BY INDICATION, 2021-2031 ($MILLION)
- TABLE 49. SPAIN MITRAL VALVE DISEASE MARKET, BY END USER, 2021-2031 ($MILLION)
- TABLE 50. REST OF EUROPE MITRAL VALVE DISEASE MARKET, BY TREATMENT TYPE, 2021-2031 ($MILLION)
- TABLE 51. REST OF EUROPE MITRAL VALVE DISEASE MARKET, BY INDICATION, 2021-2031 ($MILLION)
- TABLE 52. REST OF EUROPE MITRAL VALVE DISEASE MARKET, BY END USER, 2021-2031 ($MILLION)
- TABLE 53. ASIA-PACIFIC MITRAL VALVE DISEASE MARKET, BY TREATMENT TYPE, 2021-2031 ($MILLION)
- TABLE 54. ASIA-PACIFIC MITRAL VALVE DISEASE MARKET, BY INDICATION, 2021-2031 ($MILLION)
- TABLE 55. ASIA-PACIFIC MITRAL VALVE DISEASE MARKET, BY END USER, 2021-2031 ($MILLION)
- TABLE 56. ASIA-PACIFIC MITRAL VALVE DISEASE MARKET, BY COUNTRY, 2021-2031 ($MILLION)
- TABLE 57. JAPAN MITRAL VALVE DISEASE MARKET, BY TREATMENT TYPE, 2021-2031 ($MILLION)
- TABLE 58. JAPAN MITRAL VALVE DISEASE MARKET, BY INDICATION, 2021-2031 ($MILLION)
- TABLE 59. JAPAN MITRAL VALVE DISEASE MARKET, BY END USER, 2021-2031 ($MILLION)
- TABLE 60. CHINA MITRAL VALVE DISEASE MARKET, BY TREATMENT TYPE, 2021-2031 ($MILLION)
- TABLE 61. CHINA MITRAL VALVE DISEASE MARKET, BY INDICATION, 2021-2031 ($MILLION)
- TABLE 62. CHINA MITRAL VALVE DISEASE MARKET, BY END USER, 2021-2031 ($MILLION)
- TABLE 63. INDIA MITRAL VALVE DISEASE MARKET, BY TREATMENT TYPE, 2021-2031 ($MILLION)
- TABLE 64. INDIA MITRAL VALVE DISEASE MARKET, BY INDICATION, 2021-2031 ($MILLION)
- TABLE 65. INDIA MITRAL VALVE DISEASE MARKET, BY END USER, 2021-2031 ($MILLION)
- TABLE 66. AUSTRALIA MITRAL VALVE DISEASE MARKET, BY TREATMENT TYPE, 2021-2031 ($MILLION)
- TABLE 67. AUSTRALIA MITRAL VALVE DISEASE MARKET, BY INDICATION, 2021-2031 ($MILLION)
- TABLE 68. AUSTRALIA MITRAL VALVE DISEASE MARKET, BY END USER, 2021-2031 ($MILLION)
- TABLE 69. SOUTH KOREA MITRAL VALVE DISEASE MARKET, BY TREATMENT TYPE, 2021-2031 ($MILLION)
- TABLE 70. SOUTH KOREA MITRAL VALVE DISEASE MARKET, BY INDICATION, 2021-2031 ($MILLION)
- TABLE 71. SOUTH KOREA MITRAL VALVE DISEASE MARKET, BY END USER, 2021-2031 ($MILLION)
- TABLE 72. REST OF ASIA-PACIFIC MITRAL VALVE DISEASE MARKET, BY TREATMENT TYPE, 2021-2031 ($MILLION)
- TABLE 73. REST OF ASIA-PACIFIC MITRAL VALVE DISEASE MARKET, BY INDICATION, 2021-2031 ($MILLION)
- TABLE 74. REST OF ASIA-PACIFIC MITRAL VALVE DISEASE MARKET, BY END USER, 2021-2031 ($MILLION)
- TABLE 75. LAMEA MITRAL VALVE DISEASE MARKET, BY TREATMENT TYPE, 2021-2031 ($MILLION)
- TABLE 76. LAMEA MITRAL VALVE DISEASE MARKET, BY INDICATION, 2021-2031 ($MILLION)
- TABLE 77. LAMEA MITRAL VALVE DISEASE MARKET, BY END USER, 2021-2031 ($MILLION)
- TABLE 78. LAMEA MITRAL VALVE DISEASE MARKET, BY COUNTRY, 2021-2031 ($MILLION)
- TABLE 79. BRAZIL MITRAL VALVE DISEASE MARKET, BY TREATMENT TYPE, 2021-2031 ($MILLION)
- TABLE 80. BRAZIL MITRAL VALVE DISEASE MARKET, BY INDICATION, 2021-2031 ($MILLION)
- TABLE 81. BRAZIL MITRAL VALVE DISEASE MARKET, BY END USER, 2021-2031 ($MILLION)
- TABLE 82. SAUDI ARABIA MITRAL VALVE DISEASE MARKET, BY TREATMENT TYPE, 2021-2031 ($MILLION)
- TABLE 83. SAUDI ARABIA MITRAL VALVE DISEASE MARKET, BY INDICATION, 2021-2031 ($MILLION)
- TABLE 84. SAUDI ARABIA MITRAL VALVE DISEASE MARKET, BY END USER, 2021-2031 ($MILLION)
- TABLE 85. SOUTH AFRICA MITRAL VALVE DISEASE MARKET, BY TREATMENT TYPE, 2021-2031 ($MILLION)
- TABLE 86. SOUTH AFRICA MITRAL VALVE DISEASE MARKET, BY INDICATION, 2021-2031 ($MILLION)
- TABLE 87. SOUTH AFRICA MITRAL VALVE DISEASE MARKET, BY END USER, 2021-2031 ($MILLION)
- TABLE 88. REST OF LAMEA MITRAL VALVE DISEASE MARKET, BY TREATMENT TYPE, 2021-2031 ($MILLION)
- TABLE 89. REST OF LAMEA MITRAL VALVE DISEASE MARKET, BY INDICATION, 2021-2031 ($MILLION)
- TABLE 90. REST OF LAMEA MITRAL VALVE DISEASE MARKET, BY END USER, 2021-2031 ($MILLION)
- TABLE 91. ABBOTT LABORATORIES: KEY EXECUTIVES
- TABLE 92. ABBOTT LABORATORIES: COMPANY SNAPSHOT
- TABLE 93. ABBOTT LABORATORIES: PRODUCT SEGMENTS
- TABLE 94. ABBOTT LABORATORIES: PRODUCT PORTFOLIO
- TABLE 95. ABBOTT LABORATORIES: KEY STRATERGIES
- TABLE 96. AFFLUENT MEDICAL: KEY EXECUTIVES
- TABLE 97. AFFLUENT MEDICAL: COMPANY SNAPSHOT
- TABLE 98. AFFLUENT MEDICAL: PRODUCT SEGMENTS
- TABLE 99. AFFLUENT MEDICAL: PRODUCT PORTFOLIO
- TABLE 100. AFFLUENT MEDICAL: KEY STRATERGIES
- TABLE 101. CORCYM UK LIMITED: KEY EXECUTIVES
- TABLE 102. CORCYM UK LIMITED: COMPANY SNAPSHOT
- TABLE 103. CORCYM UK LIMITED: PRODUCT SEGMENTS
- TABLE 104. CORCYM UK LIMITED: PRODUCT PORTFOLIO
- TABLE 105. CORCYM UK LIMITED: KEY STRATERGIES
- TABLE 106. EDWARDS LIFESCIENCES CORPORATION: KEY EXECUTIVES
- TABLE 107. EDWARDS LIFESCIENCES CORPORATION: COMPANY SNAPSHOT
- TABLE 108. EDWARDS LIFESCIENCES CORPORATION: PRODUCT SEGMENTS
- TABLE 109. EDWARDS LIFESCIENCES CORPORATION: PRODUCT PORTFOLIO
- TABLE 110. EDWARDS LIFESCIENCES CORPORATION: KEY STRATERGIES
- TABLE 111. LABCOR LABORATORIOS LTDA: KEY EXECUTIVES
- TABLE 112. LABCOR LABORATORIOS LTDA: COMPANY SNAPSHOT
- TABLE 113. LABCOR LABORATORIOS LTDA: PRODUCT SEGMENTS
- TABLE 114. LABCOR LABORATORIOS LTDA: PRODUCT PORTFOLIO
- TABLE 115. MEDTRONIC PLC: KEY EXECUTIVES
- TABLE 116. MEDTRONIC PLC: COMPANY SNAPSHOT
- TABLE 117. MEDTRONIC PLC: PRODUCT SEGMENTS
- TABLE 118. MEDTRONIC PLC: PRODUCT PORTFOLIO
- TABLE 119. MEDTRONIC PLC: KEY STRATERGIES
- TABLE 120. SHOCKWAVE MEDICAL, INC.: KEY EXECUTIVES
- TABLE 121. SHOCKWAVE MEDICAL, INC.: COMPANY SNAPSHOT
- TABLE 122. SHOCKWAVE MEDICAL, INC.: PRODUCT SEGMENTS
- TABLE 123. SHOCKWAVE MEDICAL, INC.: PRODUCT PORTFOLIO
- TABLE 124. SHOCKWAVE MEDICAL, INC.: KEY STRATERGIES
- TABLE 125. VALCARE MEDICAL: KEY EXECUTIVES
- TABLE 126. VALCARE MEDICAL: COMPANY SNAPSHOT
- TABLE 127. VALCARE MEDICAL: PRODUCT SEGMENTS
- TABLE 128. VALCARE MEDICAL: PRODUCT PORTFOLIO
- TABLE 129. ARTIVION, INC.: KEY EXECUTIVES
- TABLE 130. ARTIVION, INC.: COMPANY SNAPSHOT
- TABLE 131. ARTIVION, INC.: PRODUCT SEGMENTS
- TABLE 132. ARTIVION, INC.: PRODUCT PORTFOLIO
- TABLE 133. ARTIVION, INC.: KEY STRATERGIES
- TABLE 134. BRAILE BIOMEDICA: KEY EXECUTIVES
- TABLE 135. BRAILE BIOMEDICA: COMPANY SNAPSHOT
- TABLE 136. BRAILE BIOMEDICA: PRODUCT SEGMENTS
- TABLE 137. BRAILE BIOMEDICA: PRODUCT PORTFOLIO
- TABLE 138. ZYDUS LIFESCIENCES LIMITED: KEY EXECUTIVES
- TABLE 139. ZYDUS LIFESCIENCES LIMITED: COMPANY SNAPSHOT
- TABLE 140. ZYDUS LIFESCIENCES LIMITED: PRODUCT SEGMENTS
- TABLE 141. ZYDUS LIFESCIENCES LIMITED: PRODUCT PORTFOLIO
- TABLE 142. TEVA PHARMACEUTICAL INDUSTRIES LTD.: KEY EXECUTIVES
- TABLE 143. TEVA PHARMACEUTICAL INDUSTRIES LTD.: COMPANY SNAPSHOT
- TABLE 144. TEVA PHARMACEUTICAL INDUSTRIES LTD.: PRODUCT SEGMENTS
- TABLE 145. TEVA PHARMACEUTICAL INDUSTRIES LTD.: PRODUCT PORTFOLIO
- TABLE 146. PFIZER INC.: KEY EXECUTIVES
- TABLE 147. PFIZER INC.: COMPANY SNAPSHOT
- TABLE 148. PFIZER INC.: PRODUCT SEGMENTS
- TABLE 149. PFIZER INC.: PRODUCT PORTFOLIO
- TABLE 150. BAYER AG: KEY EXECUTIVES
- TABLE 151. BAYER AG: COMPANY SNAPSHOT
- TABLE 152. BAYER AG: PRODUCT SEGMENTS
- TABLE 153. BAYER AG: PRODUCT PORTFOLIO
- TABLE 154. NOVARTIS AG: KEY EXECUTIVES
- TABLE 155. NOVARTIS AG: COMPANY SNAPSHOT
- TABLE 156. NOVARTIS AG: PRODUCT SEGMENTS
- TABLE 157. NOVARTIS AG: PRODUCT PORTFOLIO
LIST OF FIGURES
- FIGURE 01. MITRAL VALVE DISEASE MARKET, 2021-2031
- FIGURE 02. SEGMENTATION OF MITRAL VALVE DISEASE MARKET, 2021-2031
- FIGURE 03. TOP INVESTMENT POCKETS IN MITRAL VALVE DISEASE MARKET (2022-2031)
- FIGURE 04. LOW BARGAINING POWER OF SUPPLIERS
- FIGURE 05. LOW BARGAINING POWER OF BUYERS
- FIGURE 06. LOW THREAT OF SUBSTITUTES
- FIGURE 07. LOW THREAT OF NEW ENTRANTS
- FIGURE 08. LOW INTENSITY OF RIVALRY
- FIGURE 09. DRIVERS, RESTRAINTS AND OPPORTUNITIES: GLOBALMITRAL VALVE DISEASE MARKET
- FIGURE 10. MITRAL VALVE DISEASE MARKET, BY TREATMENT TYPE, 2021(%)
- FIGURE 11. COMPARATIVE SHARE ANALYSIS OF MITRAL VALVE DISEASE MARKET FOR MITRAL VALVE REPAIR, BY COUNTRY 2021 AND 2031(%)
- FIGURE 12. COMPARATIVE SHARE ANALYSIS OF MITRAL VALVE DISEASE MARKET FOR MITRAL VALVE REPLACEMENT, BY COUNTRY 2021 AND 2031(%)
- FIGURE 13. COMPARATIVE SHARE ANALYSIS OF MITRAL VALVE DISEASE MARKET FOR CARDIAC RESYNCHRONIZATION THERAPY, BY COUNTRY 2021 AND 2031(%)
- FIGURE 14. COMPARATIVE SHARE ANALYSIS OF MITRAL VALVE DISEASE MARKET FOR MITRAL VALVE THERAPEUTICS, BY COUNTRY 2021 AND 2031(%)
- FIGURE 15. MITRAL VALVE DISEASE MARKET, BY INDICATION, 2021(%)
- FIGURE 16. COMPARATIVE SHARE ANALYSIS OF MITRAL VALVE DISEASE MARKET FOR MITRAL VALVE STENOSIS, BY COUNTRY 2021 AND 2031(%)
- FIGURE 17. COMPARATIVE SHARE ANALYSIS OF MITRAL VALVE DISEASE MARKET FOR MITRAL VALVE PROLAPSE, BY COUNTRY 2021 AND 2031(%)
- FIGURE 18. COMPARATIVE SHARE ANALYSIS OF MITRAL VALVE DISEASE MARKET FOR MITRAL VALVE REGURGITATION, BY COUNTRY 2021 AND 2031(%)
- FIGURE 19. MITRAL VALVE DISEASE MARKET, BY END USER, 2021(%)
- FIGURE 20. COMPARATIVE SHARE ANALYSIS OF MITRAL VALVE DISEASE MARKET FOR HOSPITALS, BY COUNTRY 2021 AND 2031(%)
- FIGURE 21. COMPARATIVE SHARE ANALYSIS OF MITRAL VALVE DISEASE MARKET FOR AMBULATORY SURGICAL CENTERS, BY COUNTRY 2021 AND 2031(%)
- FIGURE 22. COMPARATIVE SHARE ANALYSIS OF MITRAL VALVE DISEASE MARKET FOR OTHERS, BY COUNTRY 2021 AND 2031(%)
- FIGURE 23. MITRAL VALVE DISEASE MARKET BY REGION, 2021
- FIGURE 24. U.S. MITRAL VALVE DISEASE MARKET, 2021-2031 ($MILLION)
- FIGURE 25. CANADA MITRAL VALVE DISEASE MARKET, 2021-2031 ($MILLION)
- FIGURE 26. MEXICO MITRAL VALVE DISEASE MARKET, 2021-2031 ($MILLION)
- FIGURE 27. GERMANY MITRAL VALVE DISEASE MARKET, 2021-2031 ($MILLION)
- FIGURE 28. FRANCE MITRAL VALVE DISEASE MARKET, 2021-2031 ($MILLION)
- FIGURE 29. UK MITRAL VALVE DISEASE MARKET, 2021-2031 ($MILLION)
- FIGURE 30. ITALY MITRAL VALVE DISEASE MARKET, 2021-2031 ($MILLION)
- FIGURE 31. SPAIN MITRAL VALVE DISEASE MARKET, 2021-2031 ($MILLION)
- FIGURE 32. REST OF EUROPE MITRAL VALVE DISEASE MARKET, 2021-2031 ($MILLION)
- FIGURE 33. JAPAN MITRAL VALVE DISEASE MARKET, 2021-2031 ($MILLION)
- FIGURE 34. CHINA MITRAL VALVE DISEASE MARKET, 2021-2031 ($MILLION)
- FIGURE 35. INDIA MITRAL VALVE DISEASE MARKET, 2021-2031 ($MILLION)
- FIGURE 36. AUSTRALIA MITRAL VALVE DISEASE MARKET, 2021-2031 ($MILLION)
- FIGURE 37. SOUTH KOREA MITRAL VALVE DISEASE MARKET, 2021-2031 ($MILLION)
- FIGURE 38. REST OF ASIA-PACIFIC MITRAL VALVE DISEASE MARKET, 2021-2031 ($MILLION)
- FIGURE 39. BRAZIL MITRAL VALVE DISEASE MARKET, 2021-2031 ($MILLION)
- FIGURE 40. SAUDI ARABIA MITRAL VALVE DISEASE MARKET, 2021-2031 ($MILLION)
- FIGURE 41. SOUTH AFRICA MITRAL VALVE DISEASE MARKET, 2021-2031 ($MILLION)
- FIGURE 42. REST OF LAMEA MITRAL VALVE DISEASE MARKET, 2021-2031 ($MILLION)
- FIGURE 43. TOP WINNING STRATEGIES, BY YEAR
- FIGURE 44. TOP WINNING STRATEGIES, BY DEVELOPMENT
- FIGURE 45. TOP WINNING STRATEGIES, BY COMPANY
- FIGURE 46. PRODUCT MAPPING OF TOP 10 PLAYERS
- FIGURE 47. COMPETITIVE DASHBOARD
- FIGURE 48. COMPETITIVE HEATMAP: MITRAL VALVE DISEASE MARKET
- FIGURE 49. TOP PLAYER POSITIONING, 2021
- FIGURE 50. ABBOTT LABORATORIES: NET SALES, 2020-2022 ($MILLION)
- FIGURE 51. ABBOTT LABORATORIES: REVENUE SHARE BY SEGMENT, 2022 (%)
- FIGURE 52. ABBOTT LABORATORIES: REVENUE SHARE BY REGION, 2022 (%)
- FIGURE 53. EDWARDS LIFESCIENCES CORPORATION: NET SALES, 2020-2022 ($MILLION)
- FIGURE 54. EDWARDS LIFESCIENCES CORPORATION: REVENUE SHARE BY REGION, 2021 (%)
- FIGURE 55. MEDTRONIC PLC: NET SALES, 2020-2022 ($MILLION)
- FIGURE 56. MEDTRONIC PLC: REVENUE SHARE BY SEGMENT, 2022 (%)
- FIGURE 57. MEDTRONIC PLC: REVENUE SHARE BY REGION, 2022 (%)
- FIGURE 58. SHOCKWAVE MEDICAL, INC.: NET REVENUE, 2020-2022 ($MILLION)
- FIGURE 59. SHOCKWAVE MEDICAL, INC.: REVENUE SHARE BY REGION, 2022 (%)
- FIGURE 60. ARTIVION, INC.: NET REVENUE, 2020-2022 ($MILLION)
- FIGURE 61. ARTIVION, INC.: REVENUE SHARE BY SEGMENT, 2022 (%)
- FIGURE 62. ARTIVION, INC.: REVENUE SHARE BY REGION, 2022 (%)
- FIGURE 63. ZYDUS LIFESCIENCES LIMITED: NET REVENUE, 2020-2022 ($MILLION)
- FIGURE 64. ZYDUS LIFESCIENCES LIMITED: REVENUE SHARE BY SEGMENT, 2022 (%)
- FIGURE 65. ZYDUS LIFESCIENCES LIMITED: REVENUE SHARE BY REGION, 2022 (%)
- FIGURE 66. TEVA PHARMACEUTICAL INDUSTRIES LTD.: NET REVENUE, 2020-2022 ($MILLION)
- FIGURE 67. TEVA PHARMACEUTICAL INDUSTRIES LTD.: REVENUE SHARE BY REGION, 2022 (%)
- FIGURE 68. PFIZER INC.: NET REVENUE, 2020-2022 ($MILLION)
- FIGURE 69. PFIZER INC.: REVENUE SHARE BY SEGMENT, 2022 (%)
- FIGURE 70. PFIZER INC.: REVENUE SHARE BY REGION, 2022 (%)
- FIGURE 71. BAYER AG: NET SALES, 2020-2022 ($MILLION)
- FIGURE 72. BAYER AG: REVENUE SHARE BY SEGMENT, 2022 (%)
- FIGURE 73. BAYER AG: REVENUE SHARE BY REGION, 2022 (%)
- FIGURE 74. NOVARTIS AG: NET SALES, 2020-2022 ($MILLION)
- FIGURE 75. NOVARTIS AG: REVENUE SHARE BY SEGMENT, 2022 (%)
- FIGURE 76. NOVARTIS AG: REVENUE SHARE BY REGION, 2022 (%)








