 |
市场调查报告书
商品编码
1472191
关节炎药物市场:按产品类型,按应用:2023-2032 年全球机会分析和产业预测Arthritic Therapeutic Market By Product Type (Non-steroidal Anti-inflammatory Drugs, Disease Modified Anti-rheumatoid Drugs, Biologics, Others), By Application : Global Opportunity Analysis and Industry Forecast, 2023-2032 |
||||||
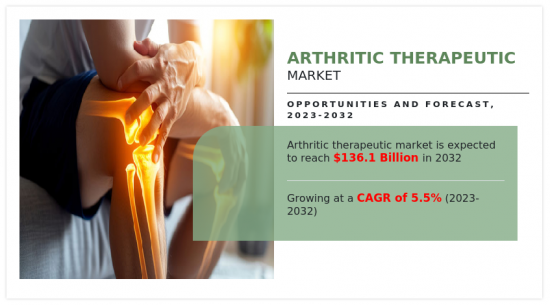
2022年关节炎药物市值为795亿美元,预计2032年将达到1,361亿美元,2023年至2032年的复合年增长率为5.5%。关节炎药物包括旨在缓解症状和治疗各种形式关节炎(包括骨关节炎和类风湿性关节炎)的全面医疗干预措施。这些治疗方法包括药物、生物製药和生活方式的改变,以缓解疼痛、减少发炎和增强关节功能。其目的是提高关节炎患者的整体生活质量,并解决关节相关疾病带来的独特挑战。关节炎治疗学是一个充满活力的领域,它整合了医学进步、个人化治疗策略和创新解决方案,为患有关节炎症状的人提供有效的缓解和增强长期福祉。
关节炎(包括骨关节炎、干癣性关节炎和类风湿性关节炎)盛行率的增加一直是推动关节炎药物市场成长的关键因素。根据美国疾病管制与预防中心 (CDC) 的数据,超过 4,700 万美国人患有关节炎,其中约 3,080 万人患有骨关节炎,130 万人类风湿性关节炎。这种不断上升的盛行率凸显了迫切需要有效的治疗性介入来控制症状、减轻疼痛和改善关节功能。随着关节炎盛行率持续上升,对关节炎治疗药物的需求增加,导致製药业的研究、开发和创新激增。医疗保健提供者和政策制定者越来越认识到解决日益严重的关节炎负担、扩大关节炎综合治疗的机会以及满足受影响者不断变化的医疗保健需求的重要性,我们正在推动旨在改进治疗方案的投资和倡议。
此外,关节炎药物市场的成长是由研发倡议的激增和大量正在研发的药物所推动的。辉瑞(Pfizer)和安进(Amgen)等大公司集中体现了这一趋势。辉瑞的类类风湿性关节炎药物Dekavi是一款处于类类风湿性关节炎2期临床试验的新药,凸显了该公司对创新解决方案的承诺。同样,安进公司的 ABP 654(一种处于 3 期临床试验的生物相似药)代表了对替代性且具有成本效益的治疗方法的追求。领先製药公司的这些重大投资不仅表明了致力于解决未满足的医疗需求,而且表明了该行业为推进关节炎患者的治疗选择而做出的共同努力。随着更多有前景的药物透过临床试验取得进展,市场有望成长,反映出加强治疗方式和改善关节炎管理结果的积极前景。
关节炎药物市场按类型、应用和地区细分。依类型划分,市场分为非类固醇消炎剂(NSAID)、缓解疾病抗风湿药(DMARD)、生物製药等。依应用,市场分为类风湿性关节炎、骨关节炎、干癣性关节炎、僵直性脊椎炎。按地区划分,我们有北美(美国、加拿大、墨西哥)、欧洲(德国、法国、英国、义大利、西班牙其他欧洲国家地区)、亚太地区(中国、日本、印度、澳洲、韩国、其他亚太地区),在洛杉矶(巴西、哥伦比亚、阿根廷和其他洛杉矶地区)和中东和非洲(海湾合作委员会、北非和其他中东和非洲地区)进行了分析。
相关人员的主要利益
- 本报告定量分析了 2022 年至 2032 年关节炎治疗市场分析的细分市场、当前趋势、估计和动态,并确定了一般关节炎治疗市场机会。
- 我们提供市场研究以及与市场驱动因素、市场限制和市场机会相关的资讯。
- 波特的五力分析强调买家和供应商帮助相关人员做出利润驱动的商业决策并加强供应商-买家网路的力量。
- 对关节炎治疗药物市场细分的详细分析有助于识别市场机会。
- 每个地区的主要国家都根据其对全球市场的收益贡献绘製了地图。
- 市场参与者定位有助于基准化分析,并提供对市场参与者当前地位的清晰了解。
- 该报告包括对区域和全球关节炎药物市场趋势、主要企业、细分市场、应用领域和市场成长策略的分析。
该报告可以定制(需要额外的费用和时间表)。
- 监管指引
- 根据客户兴趣加入公司简介
- 按国家或地区进行的附加分析 – 市场规模和预测
- 公司简介的扩充列表
- 历史市场资料
- 主要参与者的详细资料(Excel格式,包括位置、联络资讯、供应商/供应商网路等)
目录
第一章简介
第 2 章执行摘要
第三章市场概况
- 市场定义和范围
- 主要发现
- 影响因素
- 主要投资机会
- 波特五力分析
- 市场动态
- 促进因素
- 抑制因素
- 机会
第四章关节炎治疗药物市场:依产品类型
- 概述
- 非类固醇消炎剂(NSAID)
- 缓解疾病抗风湿药物 (DMARD)
- 生技药品
- 其他的
第五章关节炎药物市场:依应用分类
- 概述
- 类风湿性关节炎
- 骨关节炎
- 干癣性关节炎
- 僵直性脊椎炎
- 其他的
第六章类风湿性关节炎治疗药物市场:依地区
- 概述
- 北美洲
- 美国
- 加拿大
- 墨西哥
- 欧洲
- 德国
- 法国
- 英国
- 义大利
- 西班牙
- 其他的
- 亚太地区
- 日本
- 中国
- 印度
- 澳洲
- 韩国
- 其他的
- 拉丁美洲
- 巴西
- 哥伦比亚
- 阿根廷
- 其他拉丁美洲
- 中东/非洲
- Gcc
- 南非
- 北非
- 其他的
第七章 竞争格局
- 介绍
- 关键成功策略
- 10家主要企业产品图谱
- 竞争对手仪表板
- 竞争热图
- 2022年主要企业定位
第八章 公司简介
- AbbVie Inc.
- Pfizer Inc.
- Amgen Inc.
- Novartis AG
- Jhonson & Jhonson
- Viatris Inc.
- Bausch Health(Storz Opthalmic Instrument)
- LEO Pharma
- Merck & Co., Inc.
- Celltrion Inc
The arthritic therapeutic market was valued at $79.5 billion in 2022 and is estimated to reach $136.1 billion by 2032, exhibiting a CAGR of 5.5% from 2023 to 2032. Arthritis therapeutics involves a comprehensive array of medical interventions aimed at alleviating symptoms and managing various forms of arthritis, such as osteoarthritis and rheumatoid arthritis. These therapeutic approaches include pharmaceuticals, biologics, and lifestyle modifications tailored to mitigate pain, reduce inflammation, and enhance joint function. The goal is to improve the overall quality of life for individuals affected by arthritis, addressing the unique challenges posed by joint-related ailments. Arthritis therapeutics reflects a dynamic field that integrates medical advancements, personalized treatment strategies, and innovative solutions to provide effective relief and enhance the long-term well-being of those suffering with arthritis conditions.
The growing prevalence of arthritis conditions, including osteoarthritis, psoriatic arthritis, and rheumatoid arthritis, serves as a significant driver fueling the growth of the arthritic therapeutic market. According to the Center for Disease Control and Prevention (CDC), over 47 million Americans are affected by arthritis, with osteoarthritis impacting approximately 30.8 million individuals and rheumatoid arthritis affecting 1.3 million. This escalating prevalence underscores the pressing need for effective therapeutic interventions to manage symptoms, alleviate pain, and improve joint function. As the prevalence of arthritis conditions continues to rise, the demand for arthritis therapeutics is propelled, leading to a surge in research, development, and innovation within the pharmaceutical industry. Healthcare providers and policymakers increasingly recognize the importance of addressing the growing burden of arthritis, driving investments and initiatives aimed at expanding access to comprehensive arthritis care and advancing treatment options to meet the evolving healthcare needs of affected individuals.

In addition, the growth of the arthritic therapeutic market is propelled by a surge in R&D initiatives and a multitude of drugs in the pipeline. Major players such as Pfizer and Amgen Inc. exemplify this trend. Pfizer's Dekavi, a new molecular entity in Phase 2 trials for Rheumatoid Arthritis, underscores the commitment to innovative solutions. Similarly, Amgen's ABP 654, an investigational biosimilar in Phase 3 clinical trials, demonstrates the pursuit of alternative, cost-effective therapies. These substantial investments by key pharmaceutical companies not only showcase a dedication to addressing unmet medical needs but also indicate a collective industry effort to advance therapeutic options for arthritis patients. As more promising drugs progress through clinical trials, the market is poised for growth, reflecting a positive outlook for enhanced treatment modalities and improved outcomes in arthritis management.
The arthritic therapeutic market is segmented on the basis of type, application, and region. On the basis of type, the market is segmented into non-steroidal anti-inflammatory drugs (NSAIDs), disease modified anti-rheumatoid drugs (DMARDs), biologics and others. On the basis of application, the market is segmented into rheumatoid arthritis, osteoarthritis, psoriatic arthritis, ankylosing spondylitis, and others. Region-wise, the market is analyzed across North America (U.S., Canada, and Mexico), Europe (Germany, France, UK, Italy, Spain, and rest of Europe), Asia-Pacific (China, Japan, India, Australia, South Korea, and rest of Asia-Pacific), and LA (Brazil, Colombia, Argentina, and rest of LA) and MEA (GCC, South Africa, North Africa and rest of MEA).
The major key players that operate in the global arthritic therapeutic market are AbbVie Inc., Pfizer, Amgen Inc, Bristol-Myers Squibb, Eli Lilly and Company, F. Hoffmann-La Roche Ltd, Biogen Inc, Celltrion Healthcare Co. Ltd, Sobi Inc. The key players have adopted product launch, product development and product approval as the key strategy to expand their product portfolio.
Key Benefits for Stakeholders
- This report provides a quantitative analysis of the market segments, current trends, estimations, and dynamics of the arthritic therapeutic market analysis from 2022 to 2032 to identify the prevailing arthritic therapeutic market opportunities.
- The market research is offered along with information related to key drivers, restraints, and opportunities.
- Porter's five forces analysis highlights the potency of buyers and suppliers to enable stakeholders make profit-oriented business decisions and strengthen their supplier-buyer network.
- In-depth analysis of the arthritic therapeutic market segmentation assists to determine the prevailing market opportunities.
- Major countries in each region are mapped according to their revenue contribution to the global market.
- Market player positioning facilitates benchmarking and provides a clear understanding of the present position of the market players.
- The report includes the analysis of the regional as well as global arthritic therapeutic market trends, key players, market segments, application areas, and market growth strategies.
Additional benefits you will get with this purchase are:
- Quarterly Update and* (only available with a corporate license, on listed price)
- 5 additional Company Profile of client Choice pre- or Post-purchase, as a free update.
- Free Upcoming Version on the Purchase of Five and Enterprise User License.
- 16 analyst hours of support* (post-purchase, if you find additional data requirements upon review of the report, you may receive support amounting to 16 analyst hours to solve questions, and post-sale queries)
- 15% Free Customization* (in case the scope or segment of the report does not match your requirements, 15% is equivalent to 3 working days of free work, applicable once)
- Free data Pack on the Five and Enterprise User License. (Excel version of the report)
- Free Updated report if the report is 6-12 months old or older.
- 24-hour priority response*
- Free Industry updates and white papers.
Possible Customization with this report (with additional cost and timeline, please talk to the sales executive to know more)
- Regulatory Guidelines
- Additional company profiles with specific to client's interest
- Additional country or region analysis- market size and forecast
- Expanded list for Company Profiles
- Historic market data
- Key player details (including location, contact details, supplier/vendor network etc. in excel format)
Key Market Segments
By Product Type
- Non-steroidal Anti-inflammatory Drugs (NSAIDs)
- Disease Modified Anti-rheumatoid Drugs (DMARDs)
- Biologics
- Others
By Application
- Rheumatoid Arthritis
- Osteoarthritis
- Psoriatic Arthritis
- Ankylosing Spondylitis
- Others
By Region
- North America
- U.S.
- Canada
- Mexico
- Europe
- Germany
- France
- UK
- Italy
- Spain
- Rest of Europe
- Asia-Pacific
- Japan
- China
- India
- Australia
- South Korea
- Rest of Asia-Pacific
- Latin America
- Brazil
- Colombia
- Argentina
- Rest Of La
- Middle East and Africa
- Gcc
- South Africa
- North Africa
- Rest Of Mea
Key Market Players:
- AbbVie Inc.
- Pfizer Inc.
- Amgen Inc.
- Novartis AG
- Jhonson & Jhonson
- Viatris Inc.
- Bausch Health (Storz Opthalmic Instrument)
- LEO Pharma
- Merck & Co., Inc.
- Celltrion Inc
TABLE OF CONTENTS
CHAPTER 1: INTRODUCTION
- 1.1. Report description
- 1.2. Key market segments
- 1.3. Key benefits to the stakeholders
- 1.4. Research methodology
- 1.4.1. Primary research
- 1.4.2. Secondary research
- 1.4.3. Analyst tools and models
CHAPTER 2: EXECUTIVE SUMMARY
- 2.1. CXO perspective
CHAPTER 3: MARKET OVERVIEW
- 3.1. Market definition and scope
- 3.2. Key findings
- 3.2.1. Top impacting factors
- 3.2.2. Top investment pockets
- 3.3. Porter's five forces analysis
- 3.4. Market dynamics
- 3.4.1. Drivers
- 3.4.2. Restraints
- 3.4.3. Opportunities
CHAPTER 4: ARTHRITIC THERAPEUTIC MARKET, BY PRODUCT TYPE
- 4.1. Overview
- 4.1.1. Market size and forecast
- 4.2. Non-steroidal Anti-inflammatory Drugs (NSAIDs)
- 4.2.1. Key market trends, growth factors and opportunities
- 4.2.2. Market size and forecast, by region
- 4.2.3. Market share analysis by country
- 4.3. Disease Modified Anti-rheumatoid Drugs (DMARDs)
- 4.3.1. Key market trends, growth factors and opportunities
- 4.3.2. Market size and forecast, by region
- 4.3.3. Market share analysis by country
- 4.4. Biologics
- 4.4.1. Key market trends, growth factors and opportunities
- 4.4.2. Market size and forecast, by region
- 4.4.3. Market share analysis by country
- 4.5. Others
- 4.5.1. Key market trends, growth factors and opportunities
- 4.5.2. Market size and forecast, by region
- 4.5.3. Market share analysis by country
CHAPTER 5: ARTHRITIC THERAPEUTIC MARKET, BY APPLICATION
- 5.1. Overview
- 5.1.1. Market size and forecast
- 5.2. Rheumatoid Arthritis
- 5.2.1. Key market trends, growth factors and opportunities
- 5.2.2. Market size and forecast, by region
- 5.2.3. Market share analysis by country
- 5.3. Osteoarthritis
- 5.3.1. Key market trends, growth factors and opportunities
- 5.3.2. Market size and forecast, by region
- 5.3.3. Market share analysis by country
- 5.4. Psoriatic Arthritis
- 5.4.1. Key market trends, growth factors and opportunities
- 5.4.2. Market size and forecast, by region
- 5.4.3. Market share analysis by country
- 5.5. Ankylosing Spondylitis
- 5.5.1. Key market trends, growth factors and opportunities
- 5.5.2. Market size and forecast, by region
- 5.5.3. Market share analysis by country
- 5.6. Others
- 5.6.1. Key market trends, growth factors and opportunities
- 5.6.2. Market size and forecast, by region
- 5.6.3. Market share analysis by country
CHAPTER 6: ARTHRITIC THERAPEUTIC MARKET, BY REGION
- 6.1. Overview
- 6.1.1. Market size and forecast By Region
- 6.2. North America
- 6.2.1. Key market trends, growth factors and opportunities
- 6.2.2. Market size and forecast, by Product Type
- 6.2.3. Market size and forecast, by Application
- 6.2.4. Market size and forecast, by country
- 6.2.4.1. U.S.
- 6.2.4.1.1. Market size and forecast, by Product Type
- 6.2.4.1.2. Market size and forecast, by Application
- 6.2.4.2. Canada
- 6.2.4.2.1. Market size and forecast, by Product Type
- 6.2.4.2.2. Market size and forecast, by Application
- 6.2.4.3. Mexico
- 6.2.4.3.1. Market size and forecast, by Product Type
- 6.2.4.3.2. Market size and forecast, by Application
- 6.3. Europe
- 6.3.1. Key market trends, growth factors and opportunities
- 6.3.2. Market size and forecast, by Product Type
- 6.3.3. Market size and forecast, by Application
- 6.3.4. Market size and forecast, by country
- 6.3.4.1. Germany
- 6.3.4.1.1. Market size and forecast, by Product Type
- 6.3.4.1.2. Market size and forecast, by Application
- 6.3.4.2. France
- 6.3.4.2.1. Market size and forecast, by Product Type
- 6.3.4.2.2. Market size and forecast, by Application
- 6.3.4.3. UK
- 6.3.4.3.1. Market size and forecast, by Product Type
- 6.3.4.3.2. Market size and forecast, by Application
- 6.3.4.4. Italy
- 6.3.4.4.1. Market size and forecast, by Product Type
- 6.3.4.4.2. Market size and forecast, by Application
- 6.3.4.5. Spain
- 6.3.4.5.1. Market size and forecast, by Product Type
- 6.3.4.5.2. Market size and forecast, by Application
- 6.3.4.6. Rest of Europe
- 6.3.4.6.1. Market size and forecast, by Product Type
- 6.3.4.6.2. Market size and forecast, by Application
- 6.4. Asia-Pacific
- 6.4.1. Key market trends, growth factors and opportunities
- 6.4.2. Market size and forecast, by Product Type
- 6.4.3. Market size and forecast, by Application
- 6.4.4. Market size and forecast, by country
- 6.4.4.1. Japan
- 6.4.4.1.1. Market size and forecast, by Product Type
- 6.4.4.1.2. Market size and forecast, by Application
- 6.4.4.2. China
- 6.4.4.2.1. Market size and forecast, by Product Type
- 6.4.4.2.2. Market size and forecast, by Application
- 6.4.4.3. India
- 6.4.4.3.1. Market size and forecast, by Product Type
- 6.4.4.3.2. Market size and forecast, by Application
- 6.4.4.4. Australia
- 6.4.4.4.1. Market size and forecast, by Product Type
- 6.4.4.4.2. Market size and forecast, by Application
- 6.4.4.5. South Korea
- 6.4.4.5.1. Market size and forecast, by Product Type
- 6.4.4.5.2. Market size and forecast, by Application
- 6.4.4.6. Rest of Asia-Pacific
- 6.4.4.6.1. Market size and forecast, by Product Type
- 6.4.4.6.2. Market size and forecast, by Application
- 6.5. Latin America
- 6.5.1. Key market trends, growth factors and opportunities
- 6.5.2. Market size and forecast, by Product Type
- 6.5.3. Market size and forecast, by Application
- 6.5.4. Market size and forecast, by country
- 6.5.4.1. Brazil
- 6.5.4.1.1. Market size and forecast, by Product Type
- 6.5.4.1.2. Market size and forecast, by Application
- 6.5.4.2. Colombia
- 6.5.4.2.1. Market size and forecast, by Product Type
- 6.5.4.2.2. Market size and forecast, by Application
- 6.5.4.3. Argentina
- 6.5.4.3.1. Market size and forecast, by Product Type
- 6.5.4.3.2. Market size and forecast, by Application
- 6.5.4.4. Rest Of La
- 6.5.4.4.1. Market size and forecast, by Product Type
- 6.5.4.4.2. Market size and forecast, by Application
- 6.6. Middle East and Africa
- 6.6.1. Key market trends, growth factors and opportunities
- 6.6.2. Market size and forecast, by Product Type
- 6.6.3. Market size and forecast, by Application
- 6.6.4. Market size and forecast, by country
- 6.6.4.1. Gcc
- 6.6.4.1.1. Market size and forecast, by Product Type
- 6.6.4.1.2. Market size and forecast, by Application
- 6.6.4.2. South Africa
- 6.6.4.2.1. Market size and forecast, by Product Type
- 6.6.4.2.2. Market size and forecast, by Application
- 6.6.4.3. North Africa
- 6.6.4.3.1. Market size and forecast, by Product Type
- 6.6.4.3.2. Market size and forecast, by Application
- 6.6.4.4. Rest Of Mea
- 6.6.4.4.1. Market size and forecast, by Product Type
- 6.6.4.4.2. Market size and forecast, by Application
CHAPTER 7: COMPETITIVE LANDSCAPE
- 7.1. Introduction
- 7.2. Top winning strategies
- 7.3. Product mapping of top 10 player
- 7.4. Competitive dashboard
- 7.5. Competitive heatmap
- 7.6. Top player positioning, 2022
CHAPTER 8: COMPANY PROFILES
- 8.1. AbbVie Inc.
- 8.1.1. Company overview
- 8.1.2. Key executives
- 8.1.3. Company snapshot
- 8.1.4. Operating business segments
- 8.1.5. Product portfolio
- 8.1.6. Business performance
- 8.1.7. Key strategic moves and developments
- 8.2. Pfizer Inc.
- 8.2.1. Company overview
- 8.2.2. Key executives
- 8.2.3. Company snapshot
- 8.2.4. Operating business segments
- 8.2.5. Product portfolio
- 8.2.6. Business performance
- 8.2.7. Key strategic moves and developments
- 8.3. Amgen Inc.
- 8.3.1. Company overview
- 8.3.2. Key executives
- 8.3.3. Company snapshot
- 8.3.4. Operating business segments
- 8.3.5. Product portfolio
- 8.3.6. Business performance
- 8.3.7. Key strategic moves and developments
- 8.4. Novartis AG
- 8.4.1. Company overview
- 8.4.2. Key executives
- 8.4.3. Company snapshot
- 8.4.4. Operating business segments
- 8.4.5. Product portfolio
- 8.4.6. Business performance
- 8.4.7. Key strategic moves and developments
- 8.5. Jhonson & Jhonson
- 8.5.1. Company overview
- 8.5.2. Key executives
- 8.5.3. Company snapshot
- 8.5.4. Operating business segments
- 8.5.5. Product portfolio
- 8.5.6. Business performance
- 8.5.7. Key strategic moves and developments
- 8.6. Viatris Inc.
- 8.6.1. Company overview
- 8.6.2. Key executives
- 8.6.3. Company snapshot
- 8.6.4. Operating business segments
- 8.6.5. Product portfolio
- 8.6.6. Business performance
- 8.6.7. Key strategic moves and developments
- 8.7. Bausch Health (Storz Opthalmic Instrument)
- 8.7.1. Company overview
- 8.7.2. Key executives
- 8.7.3. Company snapshot
- 8.7.4. Operating business segments
- 8.7.5. Product portfolio
- 8.7.6. Business performance
- 8.7.7. Key strategic moves and developments
- 8.8. LEO Pharma
- 8.8.1. Company overview
- 8.8.2. Key executives
- 8.8.3. Company snapshot
- 8.8.4. Operating business segments
- 8.8.5. Product portfolio
- 8.8.6. Business performance
- 8.8.7. Key strategic moves and developments
- 8.9. Merck & Co., Inc.
- 8.9.1. Company overview
- 8.9.2. Key executives
- 8.9.3. Company snapshot
- 8.9.4. Operating business segments
- 8.9.5. Product portfolio
- 8.9.6. Business performance
- 8.9.7. Key strategic moves and developments
- 8.10. Celltrion Inc
- 8.10.1. Company overview
- 8.10.2. Key executives
- 8.10.3. Company snapshot
- 8.10.4. Operating business segments
- 8.10.5. Product portfolio
- 8.10.6. Business performance
- 8.10.7. Key strategic moves and developments
LIST OF TABLES
- TABLE 01. GLOBAL ARTHRITIC THERAPEUTIC MARKET, BY PRODUCT TYPE, 2022-2032 ($MILLION)
- TABLE 02. ARTHRITIC THERAPEUTIC MARKET FOR NON-STEROIDAL ANTI-INFLAMMATORY DRUGS (NSAIDS), BY REGION, 2022-2032 ($MILLION)
- TABLE 03. ARTHRITIC THERAPEUTIC MARKET FOR DISEASE MODIFIED ANTI-RHEUMATOID DRUGS (DMARDS), BY REGION, 2022-2032 ($MILLION)
- TABLE 04. ARTHRITIC THERAPEUTIC MARKET FOR BIOLOGICS, BY REGION, 2022-2032 ($MILLION)
- TABLE 05. ARTHRITIC THERAPEUTIC MARKET FOR OTHERS, BY REGION, 2022-2032 ($MILLION)
- TABLE 06. GLOBAL ARTHRITIC THERAPEUTIC MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 07. ARTHRITIC THERAPEUTIC MARKET FOR RHEUMATOID ARTHRITIS, BY REGION, 2022-2032 ($MILLION)
- TABLE 08. ARTHRITIC THERAPEUTIC MARKET FOR OSTEOARTHRITIS, BY REGION, 2022-2032 ($MILLION)
- TABLE 09. ARTHRITIC THERAPEUTIC MARKET FOR PSORIATIC ARTHRITIS, BY REGION, 2022-2032 ($MILLION)
- TABLE 10. ARTHRITIC THERAPEUTIC MARKET FOR ANKYLOSING SPONDYLITIS, BY REGION, 2022-2032 ($MILLION)
- TABLE 11. ARTHRITIC THERAPEUTIC MARKET FOR OTHERS, BY REGION, 2022-2032 ($MILLION)
- TABLE 12. ARTHRITIC THERAPEUTIC MARKET, BY REGION, 2022-2032 ($MILLION)
- TABLE 13. NORTH AMERICA ARTHRITIC THERAPEUTIC MARKET, BY PRODUCT TYPE, 2022-2032 ($MILLION)
- TABLE 14. NORTH AMERICA ARTHRITIC THERAPEUTIC MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 15. NORTH AMERICA ARTHRITIC THERAPEUTIC MARKET, BY COUNTRY, 2022-2032 ($MILLION)
- TABLE 16. U.S. ARTHRITIC THERAPEUTIC MARKET, BY PRODUCT TYPE, 2022-2032 ($MILLION)
- TABLE 17. U.S. ARTHRITIC THERAPEUTIC MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 18. CANADA ARTHRITIC THERAPEUTIC MARKET, BY PRODUCT TYPE, 2022-2032 ($MILLION)
- TABLE 19. CANADA ARTHRITIC THERAPEUTIC MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 20. MEXICO ARTHRITIC THERAPEUTIC MARKET, BY PRODUCT TYPE, 2022-2032 ($MILLION)
- TABLE 21. MEXICO ARTHRITIC THERAPEUTIC MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 22. EUROPE ARTHRITIC THERAPEUTIC MARKET, BY PRODUCT TYPE, 2022-2032 ($MILLION)
- TABLE 23. EUROPE ARTHRITIC THERAPEUTIC MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 24. EUROPE ARTHRITIC THERAPEUTIC MARKET, BY COUNTRY, 2022-2032 ($MILLION)
- TABLE 25. GERMANY ARTHRITIC THERAPEUTIC MARKET, BY PRODUCT TYPE, 2022-2032 ($MILLION)
- TABLE 26. GERMANY ARTHRITIC THERAPEUTIC MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 27. FRANCE ARTHRITIC THERAPEUTIC MARKET, BY PRODUCT TYPE, 2022-2032 ($MILLION)
- TABLE 28. FRANCE ARTHRITIC THERAPEUTIC MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 29. UK ARTHRITIC THERAPEUTIC MARKET, BY PRODUCT TYPE, 2022-2032 ($MILLION)
- TABLE 30. UK ARTHRITIC THERAPEUTIC MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 31. ITALY ARTHRITIC THERAPEUTIC MARKET, BY PRODUCT TYPE, 2022-2032 ($MILLION)
- TABLE 32. ITALY ARTHRITIC THERAPEUTIC MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 33. SPAIN ARTHRITIC THERAPEUTIC MARKET, BY PRODUCT TYPE, 2022-2032 ($MILLION)
- TABLE 34. SPAIN ARTHRITIC THERAPEUTIC MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 35. REST OF EUROPE ARTHRITIC THERAPEUTIC MARKET, BY PRODUCT TYPE, 2022-2032 ($MILLION)
- TABLE 36. REST OF EUROPE ARTHRITIC THERAPEUTIC MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 37. ASIA-PACIFIC ARTHRITIC THERAPEUTIC MARKET, BY PRODUCT TYPE, 2022-2032 ($MILLION)
- TABLE 38. ASIA-PACIFIC ARTHRITIC THERAPEUTIC MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 39. ASIA-PACIFIC ARTHRITIC THERAPEUTIC MARKET, BY COUNTRY, 2022-2032 ($MILLION)
- TABLE 40. JAPAN ARTHRITIC THERAPEUTIC MARKET, BY PRODUCT TYPE, 2022-2032 ($MILLION)
- TABLE 41. JAPAN ARTHRITIC THERAPEUTIC MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 42. CHINA ARTHRITIC THERAPEUTIC MARKET, BY PRODUCT TYPE, 2022-2032 ($MILLION)
- TABLE 43. CHINA ARTHRITIC THERAPEUTIC MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 44. INDIA ARTHRITIC THERAPEUTIC MARKET, BY PRODUCT TYPE, 2022-2032 ($MILLION)
- TABLE 45. INDIA ARTHRITIC THERAPEUTIC MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 46. AUSTRALIA ARTHRITIC THERAPEUTIC MARKET, BY PRODUCT TYPE, 2022-2032 ($MILLION)
- TABLE 47. AUSTRALIA ARTHRITIC THERAPEUTIC MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 48. SOUTH KOREA ARTHRITIC THERAPEUTIC MARKET, BY PRODUCT TYPE, 2022-2032 ($MILLION)
- TABLE 49. SOUTH KOREA ARTHRITIC THERAPEUTIC MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 50. REST OF ASIA-PACIFIC ARTHRITIC THERAPEUTIC MARKET, BY PRODUCT TYPE, 2022-2032 ($MILLION)
- TABLE 51. REST OF ASIA-PACIFIC ARTHRITIC THERAPEUTIC MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 52. LATIN AMERICA ARTHRITIC THERAPEUTIC MARKET, BY PRODUCT TYPE, 2022-2032 ($MILLION)
- TABLE 53. LATIN AMERICA ARTHRITIC THERAPEUTIC MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 54. LATIN AMERICA ARTHRITIC THERAPEUTIC MARKET, BY COUNTRY, 2022-2032 ($MILLION)
- TABLE 55. BRAZIL ARTHRITIC THERAPEUTIC MARKET, BY PRODUCT TYPE, 2022-2032 ($MILLION)
- TABLE 56. BRAZIL ARTHRITIC THERAPEUTIC MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 57. COLOMBIA ARTHRITIC THERAPEUTIC MARKET, BY PRODUCT TYPE, 2022-2032 ($MILLION)
- TABLE 58. COLOMBIA ARTHRITIC THERAPEUTIC MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 59. ARGENTINA ARTHRITIC THERAPEUTIC MARKET, BY PRODUCT TYPE, 2022-2032 ($MILLION)
- TABLE 60. ARGENTINA ARTHRITIC THERAPEUTIC MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 61. REST OF LA ARTHRITIC THERAPEUTIC MARKET, BY PRODUCT TYPE, 2022-2032 ($MILLION)
- TABLE 62. REST OF LA ARTHRITIC THERAPEUTIC MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 63. MIDDLE EAST AND AFRICA ARTHRITIC THERAPEUTIC MARKET, BY PRODUCT TYPE, 2022-2032 ($MILLION)
- TABLE 64. MIDDLE EAST AND AFRICA ARTHRITIC THERAPEUTIC MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 65. MIDDLE EAST AND AFRICA ARTHRITIC THERAPEUTIC MARKET, BY COUNTRY, 2022-2032 ($MILLION)
- TABLE 66. GCC ARTHRITIC THERAPEUTIC MARKET, BY PRODUCT TYPE, 2022-2032 ($MILLION)
- TABLE 67. GCC ARTHRITIC THERAPEUTIC MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 68. SOUTH AFRICA ARTHRITIC THERAPEUTIC MARKET, BY PRODUCT TYPE, 2022-2032 ($MILLION)
- TABLE 69. SOUTH AFRICA ARTHRITIC THERAPEUTIC MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 70. NORTH AFRICA ARTHRITIC THERAPEUTIC MARKET, BY PRODUCT TYPE, 2022-2032 ($MILLION)
- TABLE 71. NORTH AFRICA ARTHRITIC THERAPEUTIC MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 72. REST OF MEA ARTHRITIC THERAPEUTIC MARKET, BY PRODUCT TYPE, 2022-2032 ($MILLION)
- TABLE 73. REST OF MEA ARTHRITIC THERAPEUTIC MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 74. ABBVIE INC.: KEY EXECUTIVES
- TABLE 75. ABBVIE INC.: COMPANY SNAPSHOT
- TABLE 76. ABBVIE INC.: PRODUCT SEGMENTS
- TABLE 77. ABBVIE INC.: SERVICE SEGMENTS
- TABLE 78. ABBVIE INC.: PRODUCT PORTFOLIO
- TABLE 79. ABBVIE INC.: KEY STRATERGIES
- TABLE 80. PFIZER INC.: KEY EXECUTIVES
- TABLE 81. PFIZER INC.: COMPANY SNAPSHOT
- TABLE 82. PFIZER INC.: PRODUCT SEGMENTS
- TABLE 83. PFIZER INC.: SERVICE SEGMENTS
- TABLE 84. PFIZER INC.: PRODUCT PORTFOLIO
- TABLE 85. PFIZER INC.: KEY STRATERGIES
- TABLE 86. AMGEN INC.: KEY EXECUTIVES
- TABLE 87. AMGEN INC.: COMPANY SNAPSHOT
- TABLE 88. AMGEN INC.: PRODUCT SEGMENTS
- TABLE 89. AMGEN INC.: SERVICE SEGMENTS
- TABLE 90. AMGEN INC.: PRODUCT PORTFOLIO
- TABLE 91. AMGEN INC.: KEY STRATERGIES
- TABLE 92. NOVARTIS AG: KEY EXECUTIVES
- TABLE 93. NOVARTIS AG: COMPANY SNAPSHOT
- TABLE 94. NOVARTIS AG: PRODUCT SEGMENTS
- TABLE 95. NOVARTIS AG: SERVICE SEGMENTS
- TABLE 96. NOVARTIS AG: PRODUCT PORTFOLIO
- TABLE 97. NOVARTIS AG: KEY STRATERGIES
- TABLE 98. JHONSON & JHONSON: KEY EXECUTIVES
- TABLE 99. JHONSON & JHONSON: COMPANY SNAPSHOT
- TABLE 100. JHONSON & JHONSON: PRODUCT SEGMENTS
- TABLE 101. JHONSON & JHONSON: SERVICE SEGMENTS
- TABLE 102. JHONSON & JHONSON: PRODUCT PORTFOLIO
- TABLE 103. JHONSON & JHONSON: KEY STRATERGIES
- TABLE 104. VIATRIS INC.: KEY EXECUTIVES
- TABLE 105. VIATRIS INC.: COMPANY SNAPSHOT
- TABLE 106. VIATRIS INC.: PRODUCT SEGMENTS
- TABLE 107. VIATRIS INC.: SERVICE SEGMENTS
- TABLE 108. VIATRIS INC.: PRODUCT PORTFOLIO
- TABLE 109. VIATRIS INC.: KEY STRATERGIES
- TABLE 110. BAUSCH HEALTH (STORZ OPTHALMIC INSTRUMENT): KEY EXECUTIVES
- TABLE 111. BAUSCH HEALTH (STORZ OPTHALMIC INSTRUMENT): COMPANY SNAPSHOT
- TABLE 112. BAUSCH HEALTH (STORZ OPTHALMIC INSTRUMENT): PRODUCT SEGMENTS
- TABLE 113. BAUSCH HEALTH (STORZ OPTHALMIC INSTRUMENT): SERVICE SEGMENTS
- TABLE 114. BAUSCH HEALTH (STORZ OPTHALMIC INSTRUMENT): PRODUCT PORTFOLIO
- TABLE 115. BAUSCH HEALTH (STORZ OPTHALMIC INSTRUMENT): KEY STRATERGIES
- TABLE 116. LEO PHARMA: KEY EXECUTIVES
- TABLE 117. LEO PHARMA: COMPANY SNAPSHOT
- TABLE 118. LEO PHARMA: PRODUCT SEGMENTS
- TABLE 119. LEO PHARMA: SERVICE SEGMENTS
- TABLE 120. LEO PHARMA: PRODUCT PORTFOLIO
- TABLE 121. LEO PHARMA: KEY STRATERGIES
- TABLE 122. MERCK & CO., INC.: KEY EXECUTIVES
- TABLE 123. MERCK & CO., INC.: COMPANY SNAPSHOT
- TABLE 124. MERCK & CO., INC.: PRODUCT SEGMENTS
- TABLE 125. MERCK & CO., INC.: SERVICE SEGMENTS
- TABLE 126. MERCK & CO., INC.: PRODUCT PORTFOLIO
- TABLE 127. MERCK & CO., INC.: KEY STRATERGIES
- TABLE 128. CELLTRION INC: KEY EXECUTIVES
- TABLE 129. CELLTRION INC: COMPANY SNAPSHOT
- TABLE 130. CELLTRION INC: PRODUCT SEGMENTS
- TABLE 131. CELLTRION INC: SERVICE SEGMENTS
- TABLE 132. CELLTRION INC: PRODUCT PORTFOLIO
- TABLE 133. CELLTRION INC: KEY STRATERGIES
LIST OF FIGURES
- FIGURE 01. ARTHRITIC THERAPEUTIC MARKET, 2022-2032
- FIGURE 02. SEGMENTATION OF ARTHRITIC THERAPEUTIC MARKET,2022-2032
- FIGURE 03. TOP IMPACTING FACTORS IN ARTHRITIC THERAPEUTIC MARKET
- FIGURE 04. TOP INVESTMENT POCKETS IN ARTHRITIC THERAPEUTIC MARKET (2023-2032)
- FIGURE 05. BARGAINING POWER OF SUPPLIERS
- FIGURE 06. BARGAINING POWER OF BUYERS
- FIGURE 07. THREAT OF SUBSTITUTION
- FIGURE 08. THREAT OF SUBSTITUTION
- FIGURE 09. COMPETITIVE RIVALRY
- FIGURE 10. GLOBAL ARTHRITIC THERAPEUTIC MARKET:DRIVERS, RESTRAINTS AND OPPORTUNITIES
- FIGURE 11. ARTHRITIC THERAPEUTIC MARKET, BY PRODUCT TYPE, 2022 AND 2032(%)
- FIGURE 12. COMPARATIVE SHARE ANALYSIS OF ARTHRITIC THERAPEUTIC MARKET FOR NON-STEROIDAL ANTI-INFLAMMATORY DRUGS (NSAIDS), BY COUNTRY 2022 AND 2032(%)
- FIGURE 13. COMPARATIVE SHARE ANALYSIS OF ARTHRITIC THERAPEUTIC MARKET FOR DISEASE MODIFIED ANTI-RHEUMATOID DRUGS (DMARDS), BY COUNTRY 2022 AND 2032(%)
- FIGURE 14. COMPARATIVE SHARE ANALYSIS OF ARTHRITIC THERAPEUTIC MARKET FOR BIOLOGICS, BY COUNTRY 2022 AND 2032(%)
- FIGURE 15. COMPARATIVE SHARE ANALYSIS OF ARTHRITIC THERAPEUTIC MARKET FOR OTHERS, BY COUNTRY 2022 AND 2032(%)
- FIGURE 16. ARTHRITIC THERAPEUTIC MARKET, BY APPLICATION, 2022 AND 2032(%)
- FIGURE 17. COMPARATIVE SHARE ANALYSIS OF ARTHRITIC THERAPEUTIC MARKET FOR RHEUMATOID ARTHRITIS, BY COUNTRY 2022 AND 2032(%)
- FIGURE 18. COMPARATIVE SHARE ANALYSIS OF ARTHRITIC THERAPEUTIC MARKET FOR OSTEOARTHRITIS, BY COUNTRY 2022 AND 2032(%)
- FIGURE 19. COMPARATIVE SHARE ANALYSIS OF ARTHRITIC THERAPEUTIC MARKET FOR PSORIATIC ARTHRITIS, BY COUNTRY 2022 AND 2032(%)
- FIGURE 20. COMPARATIVE SHARE ANALYSIS OF ARTHRITIC THERAPEUTIC MARKET FOR ANKYLOSING SPONDYLITIS, BY COUNTRY 2022 AND 2032(%)
- FIGURE 21. COMPARATIVE SHARE ANALYSIS OF ARTHRITIC THERAPEUTIC MARKET FOR OTHERS, BY COUNTRY 2022 AND 2032(%)
- FIGURE 22. ARTHRITIC THERAPEUTIC MARKET BY REGION, 2022 AND 2032(%)
- FIGURE 23. U.S. ARTHRITIC THERAPEUTIC MARKET, 2022-2032 ($MILLION)
- FIGURE 24. CANADA ARTHRITIC THERAPEUTIC MARKET, 2022-2032 ($MILLION)
- FIGURE 25. MEXICO ARTHRITIC THERAPEUTIC MARKET, 2022-2032 ($MILLION)
- FIGURE 26. GERMANY ARTHRITIC THERAPEUTIC MARKET, 2022-2032 ($MILLION)
- FIGURE 27. FRANCE ARTHRITIC THERAPEUTIC MARKET, 2022-2032 ($MILLION)
- FIGURE 28. UK ARTHRITIC THERAPEUTIC MARKET, 2022-2032 ($MILLION)
- FIGURE 29. ITALY ARTHRITIC THERAPEUTIC MARKET, 2022-2032 ($MILLION)
- FIGURE 30. SPAIN ARTHRITIC THERAPEUTIC MARKET, 2022-2032 ($MILLION)
- FIGURE 31. REST OF EUROPE ARTHRITIC THERAPEUTIC MARKET, 2022-2032 ($MILLION)
- FIGURE 32. JAPAN ARTHRITIC THERAPEUTIC MARKET, 2022-2032 ($MILLION)
- FIGURE 33. CHINA ARTHRITIC THERAPEUTIC MARKET, 2022-2032 ($MILLION)
- FIGURE 34. INDIA ARTHRITIC THERAPEUTIC MARKET, 2022-2032 ($MILLION)
- FIGURE 35. AUSTRALIA ARTHRITIC THERAPEUTIC MARKET, 2022-2032 ($MILLION)
- FIGURE 36. SOUTH KOREA ARTHRITIC THERAPEUTIC MARKET, 2022-2032 ($MILLION)
- FIGURE 37. REST OF ASIA-PACIFIC ARTHRITIC THERAPEUTIC MARKET, 2022-2032 ($MILLION)
- FIGURE 38. BRAZIL ARTHRITIC THERAPEUTIC MARKET, 2022-2032 ($MILLION)
- FIGURE 39. COLOMBIA ARTHRITIC THERAPEUTIC MARKET, 2022-2032 ($MILLION)
- FIGURE 40. ARGENTINA ARTHRITIC THERAPEUTIC MARKET, 2022-2032 ($MILLION)
- FIGURE 41. REST OF LA ARTHRITIC THERAPEUTIC MARKET, 2022-2032 ($MILLION)
- FIGURE 42. GCC ARTHRITIC THERAPEUTIC MARKET, 2022-2032 ($MILLION)
- FIGURE 43. SOUTH AFRICA ARTHRITIC THERAPEUTIC MARKET, 2022-2032 ($MILLION)
- FIGURE 44. NORTH AFRICA ARTHRITIC THERAPEUTIC MARKET, 2022-2032 ($MILLION)
- FIGURE 45. REST OF MEA ARTHRITIC THERAPEUTIC MARKET, 2022-2032 ($MILLION)
- FIGURE 46. TOP WINNING STRATEGIES, BY YEAR
- FIGURE 47. TOP WINNING STRATEGIES, BY DEVELOPMENT
- FIGURE 48. TOP WINNING STRATEGIES, BY COMPANY
- FIGURE 49. PRODUCT MAPPING OF TOP 10 PLAYERS
- FIGURE 50. COMPETITIVE DASHBOARD
- FIGURE 51. COMPETITIVE HEATMAP: ARTHRITIC THERAPEUTIC MARKET
- FIGURE 52. TOP PLAYER POSITIONING, 2022





