 |
市场调查报告书
商品编码
1358053
血栓除去装置市场:按产品类型、按用途、按最终用户、按地区Thrombectomy Devices Market, By Product Type, By Application, By End User, By Geography |
||||||
2023年,全球血栓除去装置市场估计为16.8亿美元,预计在预测期内(2023-2030年)年复合成长率为7.5%。
| 报告范围 | 报告详情 | ||
|---|---|---|---|
| 基准年 | 2022年 | 2023年市场规模 | 16.8亿美元 |
| 实绩资料 | 2018-2021 | 预测期 | 2023-2030 |
| 预测期年复合成长率 | 7.50% | 2030年市场规模预测 | 27.8亿美元 |
血栓切除术是指以物理方式移除血管内的软血块。血栓除去装置易于使用,能够持续打开血管并清除血栓。使用血栓除去装置可以透过提高手术再通率来改善长期神经系统结果,例如缺血性脑损伤。一旦再通开始,血栓就会软化并部分溶解,表示临床恢復。血栓除去装置可用于溶栓治疗等药物治疗无效或不适当的患者、最近接受心血管手术的患者或正在服用口服抗凝血剂的患者。机械抽吸血栓除去装置是双腔导管,使用标准 0.014 英吋导丝穿过病变病变。两个管腔中较窄的管腔结构导丝,并使用快速替换单轨系统。週边血栓切除术用于治疗周边动脉疾病(PAD)、急性肢体缺血(ALI)、严重肢体缺血(CLI)、慢性完全阻塞(CTO)、深层静脉栓塞症(DVT)和肺动脉栓塞(PE) .其他末稍血管疾病(PVD)。
市场动态
美国食品药物管理局等法规机构对产品核可增加预计将在预测期内推动全球血栓除去装置市场的成长。例如,2020年5月,医疗技术公司Control Medical Technology, LLC核准其Aspire MAX 7-11F机械血栓清除系统已获得美国食品药物管理局批准,该系统旨在清除末稍血管中的血栓。 Aspire MAX 7-11F 机械血栓切除系统包括 Aspire 吸引器和/或电子机械泵驱动的新型大口径、弹性、抗扭结导管 (20)。
本研究的主要特点
- 该研究报告对全球血栓除去装置市场进行了深入分析,并提供了以2022年为基准年的预测期(2023-2030年)的市场规模和年复合成长率(CAGR)。它揭示了各个细分市场的潜在商机,并说明了该市场有吸引力的投资提案矩阵。
- 它还提供了有关市场促进因素、抑制因素、机会、新产品发布和核准、市场趋势、区域前景、主要企业采取的竞争策略等的主要考察。
- 它根据公司亮点、产品系列、主要亮点、绩效和策略等参数,介绍了全球血栓除去装置市场的主要企业。
- 该报告的见解使行销人员和公司负责人能够就未来的产品发布、类型升级、市场经营团队和行销策略做出明智的资讯。
- 全球血栓切除设备市场报告针对该行业的各个相关人员,如投资者、供应商、产品製造商、经销商、新进业者和财务分析师。
- 透过用于分析全球血栓切除设备市场的各种策略矩阵,将促进相关人员的决策。
目录
第1章调查目的和假设
- 这项研究的目的
- 假设
- 简称
第2章市场展望
- 报告说明
- 市场定义和范围
- 执行摘要
- Coherent Opportunity Map(COM)
第3章市场动态、法规及趋势分析
- 市场动态
- 促进因素
- 抑制因素
- 市场机会
- 影响分析
- 最近的产品核准/发布
- 合併、收购和合作
- 法规场景
- 价格分析
- 主要进展
- PEST分析
- 波特的分析
第4章全球血栓除去装置市场-新型冠状病毒感染疾病(COVID-19)的影响分析
- 经济影响
- 新型冠状病毒感染疾病(COVID-19)的流行病学
- 对需求和供给的影响
第5章全球血栓除去装置市场:依产品类型,2018-2030
- 机械取血栓除去装置
- 抽吸式血栓除去装置
- 动态血栓除去装置
- 超音波血栓除去装置
第6章全球血栓除去装置市场:依用途,2018-2030
- 深层静脉栓塞症(DVT)
- 肺动脉栓塞(PE)
- 浅静脉血栓症形成
- 肾静脉血栓症(RVT)
- 动脉血栓症(血栓症)
- 其他(如缺血性中风)
第7章全球血栓除去装置市场:按最终用户划分,2018-2030 年
- 医院
- 门诊手术中心
- 其他(学术研究机构等)
第8章全球血栓除去装置市场:按地区划分,2018-2030 年
- 北美洲
- 美国
- 加拿大
- 欧洲
- 英国
- 德国
- 义大利
- 法国
- 西班牙
- 俄罗斯
- 其他欧洲国家
- 亚太地区
- 中国
- 印度
- 日本
- ASEAN
- 澳洲
- 韩国
- 其他亚太地区
- 拉丁美洲
- 巴西
- 墨西哥
- 阿根廷
- 其他拉丁美洲
- 中东
- GCC
- 以色列
- 其他中东地区
- 非洲
- 北非
- 中部非洲
- 南非
第9章竞争形势
- 公司简介
- Boston Scientific Corporation
- Medtronic
- Merit Medical System Inc.
- Stryker Corporation
- Terumo Corporation
- Teleflex Incorporated
- Vetex Medical Ltd.
- Edwards Lifesciences Corporation
- Penumbra Inc.
- Control Medical Technology, LLC.
- Rapid Medical
- Abbott
- Surmodics Inc.
- Analysts'Views
第10章章
- 参考
- 调查方法
Global thrombectomy devices market is estimated to be valued at US$ 1.68 Bn in 2023, and is expected to exhibit a CAGR of 7.5% during the forecast period (2023-2030).
| Report Coverage | Report Details | ||
|---|---|---|---|
| Base Year: | 2022 | Market Size in 2023: | US$ 1.68 Bn |
| Historical Data for: | 2018 to 2021 | Forecast Period: | 2023 - 2030 |
| Forecast Period 2023 to 2030 CAGR: | 7.50% | 2030 Value Projection: | US$ 2.78 Bn |

Thrombectomy refers to physical removal of soft thrombus from blood vessels. A thrombectomy device is easy to use, and it consistently opens blood vessels for the removal of blood clots. Use of thrombectomy devices can improve long-term neurological outcomes such as ischemic brain injury and others, by providing higher rates of procedural recanalization. Once recanalization starts, clot softens and partially dissolves, indicating clinical recovery. Thrombectomy devices can be used in patients, where pharmacological treatments such as thrombolysis are likely to be ineffective or inappropriate, in case of recent cardiovascular surgery or in patients taking oral anticoagulants. Mechanical aspiration thrombectomy devices are dual-lumen catheters that are passed across the culprit lesion over a standard 0.014-inch guidewire. Of the two lumens, the smaller lumen consists of the guidewire by using a rapid exchange monorail system. Peripheral thrombectomy is associated with peripheral arterial disease (PAD), acute limb ischemia (ALI), critical limb ischemia (CLI), chronic total occlusion (CTO), deep vein thrombosis (DVT), pulmonary embolism (PE), and other peripheral vascular disease (PVD).
Market Dynamics
Increasing product approvals by regulatory authorities such as U.S. Food and Drug Administration is expected to drive the global thrombectomy devices market growth over the forecast period. For instance, in May 2020, Control Medical Technology, LLC., a medical technology company, announced that the U.S. Food and Drug Administration had approved Aspire MAX 7 - 11F mechanical thrombectomy system that is designed to remove blood clots from the peripheral vessels. The Aspire MAX 7 - 11F mechanical thrombectomy system includes (20) new large-lumen, flexible, and kink-resistant catheters w/dilators powered by the Aspire Aspirator and/or an electromechanical pump.
Key features of the study:
- This report provides an in-depth analysis of the global thrombectomy devices market and provides market size (US$ Bn) and compound annual growth rate (CAGR) for the forecast period (2023-2030), considering 2022 as the base year It elucidates potential revenue opportunities across different segments and explains attractive investment proposition matrices for this market.
- This study also provides key insights about market drivers, restraints, opportunities, new product launches or approval, market trends, regional outlook, and competitive strategies adopted by key players.
- It profiles key players in the global thrombectomy y devices market based on the following parameters- company highlights, products portfolio, key highlights, financial performance, and strategies.
- Key companies covered as a part of this study include Boston Scientific Corporation, Medtronic, Merit Medical System Inc., Stryker Corporation, Terumo Corporation, Teleflex Incorporated, Vetex Medical Ltd., Edwards Lifesciences Corporation, Penumbra Inc., Control Medical Technology, LLC., Rapid Medical, Abbott and Surmodics, Inc.
- Insights from this report would allow marketers and the management authorities of the companies to make informed decisions regarding their future product launches, type up-gradation, market expansion, and marketing tactics
- Global thrombectomy devices market report caters to various stakeholders in this industry including investors, suppliers, product manufacturers, distributors, new entrants, and financial analysts
- Stakeholders would have ease in decision-making through various strategy matrices used in analyzing the global thrombectomy devices market
Thrombectomy Devices Market Detailed Segmentation:
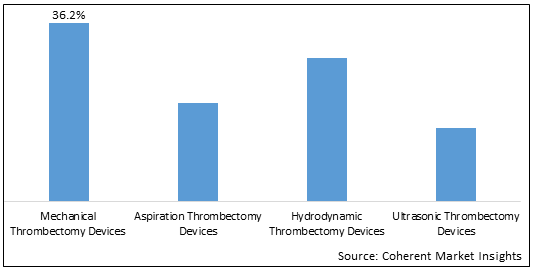
- By Product Type:
- Mechanical Thrombectomy Devices
- Aspiration Thrombectomy Devices
- Hydrodynamic Thrombectomy Devices
- Ultrasonic Thrombectomy Devices
- By Application:
- Deep Vein Thrombosis (DVT)
- Pulmonary Embolism (PE)
- Superficial Vein Thrombosis
- Renal Vein Thrombosis (RVT)
- Arterial Thrombosis (Atherothrombosis)
- Others (Ischemic Stroke, etc.)
- By End User:
- Hospitals
- Ambulatory Surgical Centers
- Others (Academic and Research Institutes, etc)
- By Region:
- North America
- Latin America
- Europe
- Asia Pacific
- Middle East
- Africa
- Company Profiles
- Boston Scientific Corporation
- Medtronic
- Merit Medical System Inc.
- Stryker Corporation
- Terumo Corporation
- Teleflex Incorporated
- Vetex Medical Ltd.
- Edwards Lifesciences Corporation
- Penumbra Inc.
- Control Medical Technology, LLC.
- Rapid Medical
- Abbott
- Surmodics, Inc.
Table of Contents
1. Research Objective and Assumption
- Research Objectives
- Assumptions
- Abbreviations
2. Market Purview
- Report Description
- Market Definition and Scope
- Executive Summary
- Market Snippet, By Product Type
- Market Snippet, By Application
- Market Snippet, By End User
- Market Snippet, By Region
- Coherent Opportunity Map (COM)
3. Market Dynamics, Regulations, and Trends Analysis
- Market Dynamics
- Drivers
- Restraints
- Market Opportunities
- Impact Analysis
- Recent Product Approvals/Launches
- Mergers, Acquisitions, and Collaborations
- Regulatory Scenario
- Pricing Analysis
- Key Developments
- PEST Analysis
- Porter's Analysis
4. Global Thrombectomy Devices Market - COVID-19 Impact Analysis
- Economic Impact
- COVID-19 Epidemiology
- Impact on Supply and Demand
5. Global Thrombectomy Devices Market, By Product Type, 2018- 2030, (US$ Bn)
- Introduction
- Market Share Analysis, 2023 and 2030 (%)
- Y-o-Y Growth Analysis, 2018-2030
- Segment Trends
- Mechanical Thrombectomy Devices
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2018 - 2030, (US$ Bn)
- Segment Trends
- Aspiration Thrombectomy Devices
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2018 - 2030, (US$ Bn)
- Segment Trends
- Hydrodynamic Thrombectomy Devices
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2018 - 2030, (US$ Bn)
- Segment Trends
- Ultrasonic Thrombectomy Devices
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2018 - 2030, (US$ Bn)
- Segment Trends
6. Global Thrombectomy Devices Market, By Application, 2018 - 2030, (US$ Bn)
- Introduction
- Market Share Analysis, 2023 and 2030 (%)
- Y-o-Y Growth Analysis, 2018-2030
- Segment Trends
- Deep Vein Thrombosis (DVT)
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2018 - 2030, (US$ Bn)
- Segment Trends
- Pulmonary Embolism (PE)
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2018 - 2030, (US$ Bn)
- Segment Trends
- Superficial Vein Thrombosis
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2018 - 2030, (US$ Bn)
- Segment Trends
- Renal Vein Thrombosis (RVT)
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2018 - 2030, (US$ Bn)
- Segment Trends
- Arterial Thrombosis (Atherothrombosis)
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2018 - 2030, (US$ Bn)
- Segment Trends
- Others (Ischemic Stroke, etc.)
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2018 - 2030, (US$ Bn)
- Segment Trends
7. Global Thrombectomy Devices Market, By End User, 2018 - 2030, (US$ Bn)
- Introduction
- Market Share Analysis, 2023 and 2030 (%)
- Y-o-Y Growth Analysis, 2018-2030
- Segment Trends
- Hospitals
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2018 - 2030, (US$ Bn)
- Ambulatory Surgical Centers
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2018 - 2030, (US$ Bn)
- Others (Academic and Research Institutes, etc.)
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2018 - 2030, (US$ Bn)
8. Global Thrombectomy Devices Market, By Region, 2018 - 2030, (US$ Bn)
- Introduction
- Market Share Analysis, 2023 and 2030 (%)
- Y-o-Y Growth Analysis, 2018-2030
- Segment Trends
- North America
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, By Product Type, 2018 -2030, (US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Application, 2018 -2030, (US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By End User, 2018-2030, (US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Country, 2018 -2030, (US$ Bn)
- U.S.
- Canada
- Europe
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, By Product Type, 2018 -2030, (US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Application, 2018 -2030, (US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By End User, 2018-2030, (US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Country, 2018 -2030, (US$ Bn)
- U.K.
- Germany
- Italy
- France
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- Introduction
- Market Size and Forecast, and Y-o-Y Growth. By Product Type, 2018 -2030, (US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Application, 2018 -2030, (US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By End User, 2018-2030, (US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Country, 2018 -2030, (US$ Bn)
- China
- India
- Japan
- ASEAN
- Australia
- South Korea
- Rest of Asia Pacific
- Latin America
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, By Product Type, 2018 -2030, (US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Application, 2018 -2030, (US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth. By End User, 2018-2030, (US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Country, 2018 -2030, (US$ Bn)
- Brazil
- Mexico
- Argentina
- Rest of Latin America
- Middle East
- Introduction
- Market Size and Forecast, and Y-o-Y Growth By Product Type, 2018 -2030, (US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth By Application, 2018 -2030, (US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth By End User, 2018-2030, (US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth By Country, 2018 -2030, (US$ Bn)
- GCC
- Israel
- Rest of Middle East
- Africa
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, By Product Type, 2018 -2030, (US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Application, 2018 -2030, (US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By End User, 2018-2030, (US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Country/Region, 2018 -2030, (US$ Bn)
- North Africa
- Central Africa
- South Africa
9. Competitive Landscape
- Company Profiles
- Boston Scientific Corporation
- Company Overview
- Product Portfolio
- Key Highlights
- Financial Overview
- Strategies
- Medtronic
- Company Overview
- Product Portfolio
- Key Highlights
- Financial Overview
- Strategies
- Merit Medical System Inc.
- Company Overview
- Product Portfolio
- Key Highlights
- Financial Overview
- Strategies
- Stryker Corporation
- Company Overview
- Product Portfolio
- Key Highlights
- Financial Overview
- Strategies
- Terumo Corporation
- Company Overview
- Product Portfolio
- Key Highlights
- Financial Overview
- Strategies
- Teleflex Incorporated
- Company Overview
- Product Portfolio
- Key Highlights
- Financial Overview
- Strategies
- Vetex Medical Ltd.
- Company Overview
- Product Portfolio
- Key Highlights
- Financial Overview
- Strategies
- Edwards Lifesciences Corporation
- Company Overview
- Product Portfolio
- Key Highlights
- Financial Overview
- Strategies
- Penumbra Inc.
- Company Overview
- Product Portfolio
- Key Highlights
- Financial Overview
- Strategies
- Control Medical Technology, LLC.
- Company Overview
- Product Portfolio
- Key Highlights
- Financial Overview
- Strategies
- Rapid Medical
- Company Overview
- Product Portfolio
- Key Highlights
- Financial Overview
- Strategies
- Abbott
- Company Overview
- Product Portfolio
- Key Highlights
- Financial Overview
- Strategies
- Surmodics Inc.
- Company Overview
- Product Portfolio
- Key Highlights
- Financial Overview
- Strategies
- Analysts' Views
10. Section
- References
- Research Methodology
- About us and Sales Contact









