 |
市场调查报告书
商品编码
1672840
eTMF(电子临床实验试验文件)市场按部署模式、功能、最终用户和地区划分Electronic Trial Master File (eTMF) Market, By Deployment Mode, By Functionality, By End User, By Geography |
||||||
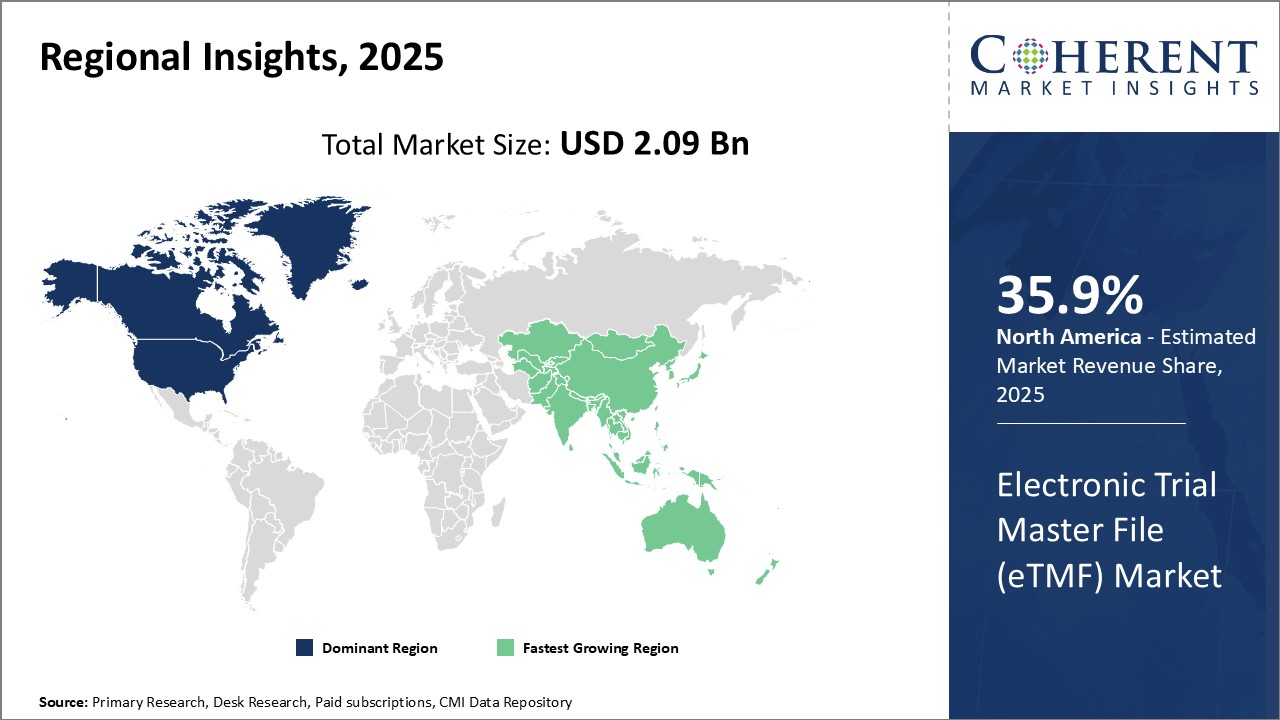
预计到 2025 年全球 eTMF(电子临床实验试验文件)市场规模将达到 20.9 亿美元,到 2032 年预计将达到 48.1 亿美元,2025 年至 2032 年的复合年增长率为 12.6%。
| 报告范围 | 报告详细信息 | ||
|---|---|---|---|
| 基准年 | 2024 | 2025 年市场规模 | 20.9亿美元 |
| 效能资料 | 从 2020 年到 2024 年 | 预测期 | 2025 至 2032 年 |
| 预测期:2025-2032年复合年增长率: | 12.60% | 2032 年价值预测 | 48.1亿美元 |
全球 eTMF(电子临床实验文件)市场的成长是由跨产业临床试验流程的扩展所推动的。 eTMF 是一个整合系统,它以数位/电子格式管理临床试验相关文件,以便于文件搜寻和存檔。组织关键的临床文献并提高整个试验生命週期的可见度。随着临床试验过程变得越来越复杂,法规变得越来越严格,进行临床试验的组织将对 eTMF 解决方案的需求很高,以确保合规性和加强监督。市场成长受到监管合规要求、eTMF 的成本效益以及药物开发研发投资增加等因素的推动。
市场动态:
有关临床试验文件和资料管理的严格监管指南可能会推动全球各地区 eTMF(电子临床实验试验文件)市场的成长。监管机构非常重视透过电子存檔进行适当的文件管理以确保资料的完整性。製药和生物技术公司增加用于开发新药的研究和开发支出也可能推动市场成长。然而,eTMF 系统实施成本高,尤其是对于中小型企业而言,可能会阻碍市场成长。 eTMF 的成本效益以及文件安全性和可访问性的提高可能会带来新的成长机会。供应商也正在转向可自订技术和云端基础的解决方案,以进一步提高可访问性并降低营运成本。
本研究的主要特点
本报告对全球 eTMF(电子临床实验试验文件)市场进行了详细分析,并以 2024 年为基准年,展示了预测期(2025-2032 年)的市场规模和復合年增长率。
它还强调了各个领域的潜在商机并说明了该市场的有吸引力的投资提案矩阵。
它还提供了对市场驱动因素、限制因素、机会、新产品发布和核准、市场趋势、区域前景以及主要企业采用的竞争策略的重要见解。
全球 eTMF(电子临床实验试验文件)市场中的主要企业是根据公司亮点、产品系列、关键亮点、财务表现和策略等参数进行介绍的。
研究涉及的主要企业包括 Veeva Systems Inc.、Medidata Solutions, Inc.、Oracle Corporation、Parexel International Corporation、IBM Watson Health、DrugDev(现为 Veeva 的一部分)、MasterControl, Inc.、ArisGlobal LLC、Dassault Systemes、Trial Interactive、Signant Health、Forte Systems, Inc., Inc.
本报告的见解将使负责人和企业经营团队能够就未来产品发布、新兴趋势、市场扩张和行销策略做出明智的决策。
全球 eTMF(电子临床实验试验文件)市场报告迎合了该行业的各个相关人员,例如投资者、供应商、产品製造商、经销商、新进业者和金融分析师。
相关人员可以透过分析全球 eTMF(电子临床实验试验文件)市场所使用的各种策略矩阵来简化决策。
目录
第一章 调查目的与前提条件
- 研究目标
- 先决条件
- 简称
第二章 市场展望
- 报告描述
- 市场定义和范围
- 执行摘要
第三章市场动态、法规与趋势分析
- 市场动态
- 驱动程式
- 限制因素
- 市场机会
- 监管情景
- 产业趋势
- 合併和收购
- 新系统实施/核准
- 新冠肺炎疫情的影响
4. 2020 年至 2032 年按部署模式分類的全球临床实验市场
- 云端基础
- 本地
5. 2020 年至 2032 年全球临床实验市场(依功能划分)
- 文件管理
- 工作流程管理
- 报告和分析
- 其他的
6. 2020 年至 2032 年全球临床实验市场(依最终用户划分)
- 製药公司
- 生技公司
- 受託研究机构(CRO)
- 学术研究所
7. 2020 年至 2032 年全球临床实验市场(按地区)
- 北美洲
- 欧洲
- 亚太地区
- 拉丁美洲
- 中东
- 非洲
第八章 竞争格局
- 公司简介
- Veeva Systems Inc.
- Medidata Solutions, Inc.
- Oracle Corporation
- Parexel International Corporation
- IBM Watson Health
- DrugDev(now part of Veeva)
- MasterControl, Inc.
- ArisGlobal LLC
- Dassault Systemes
- Trial Interactive
- Signant Health
- Forte Research Systems, Inc.
- Axiom Real-Time Metrics
- eClinical Solutions, LLC
- Bioclinica, Inc.
第九章分析师建议
- 机会
- 分析师观点
- Coherent Opportunity Map
第十章调查方法
- 参考
- 调查方法
Global Electronic Trial Master File (eTMF) Market is estimated to be valued at USD 2.09 Bn in 2025 and is expected to reach USD 4.81 Bn by 2032, growing at a compound annual growth rate (CAGR) of 12.6% from 2025 to 2032.
| Report Coverage | Report Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 2.09 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 12.60% | 2032 Value Projection: | USD 4.81 Bn |

Global electronic trial master file (eTMF) market growth is driven by growing clinical trial processes across industries. eTMF refers to an integrated system that facilitates management of documents related to clinical trials in a digital/electronic format designed for ease of retrieval and archiving of documents. It helps maintain crucial trial-related documents in an organized manner and improve visibility throughout the lifecycle of a clinical trial. With increasing complexities in clinical trial processes and stricter regulations, there will be huge demand for eTMF solutions among organizations conducting trials to ensure compliance and improved oversight. The market growth is driven by factors such as regulatory compliance requirements, cost-efficiency of eTMF, and increasing R&D investments in drug development.
Market Dynamics:
Stringent regulatory guidelines around clinical trial documentation and data management across regions can drive the global electronic trial master file (eTMF) market growth. Regulatory bodies emphasize on maintaining proper documentation with electronic archiving to ensure data integrity. Rising R&D expenditure of pharmaceutical and biotechnology companies on new drug development can also drive the market growth. However, high implementation costs of eTMF systems especially for small and mid-sized companies can hamper the market growth. Cost benefits of eTMF along with better document security and accessibility can offer new growth opportunities. Vendors are also focusing on customizable technologies and cloud-based solutions to further enhance accessibility and lower operational costs.
Key features of the study:
This report provides in-depth analysis of the global electronic trial master file (eTMF) market, and provides market size (US$ Bn) and compound annual growth rate (CAGR%) for the forecast period (2025-2032), considering 2024 as the base year
It elucidates potential revenue opportunities across different segments and explains attractive investment proposition matrices for this market
This study also provides key insights about market drivers, restraints, opportunities, new product launches or approval, market trends, regional outlook, and competitive strategies adopted by key players
It profiles key players in the global electronic trial master file (eTMF) market based on the following parameters - company highlights, products portfolio, key highlights, financial performance, and strategies
Key companies covered as a part of this study include Veeva Systems Inc., Medidata Solutions, Inc., Oracle Corporation, Parexel International Corporation, IBM Watson Health, DrugDev (now part of Veeva), MasterControl, Inc., ArisGlobal LLC, Dassault Systemes, Trial Interactive, Signant Health, Forte Research Systems, Inc., Axiom Real-Time Metrics, eClinical Solutions, LLC, and Bioclinica, Inc.
Insights from this report would allow marketers and the management authorities of the companies to make informed decisions regarding their future product launches, type up-gradation, market expansion, and marketing tactics
Global electronic trial master file (eTMF) market report caters to various stakeholders in this industry including investors, suppliers, product manufacturers, distributors, new entrants, and financial analysts
Stakeholders would have ease in decision-making through various strategy matrices used in analyzing the global electronic trial master file (eTMF) market
Detailed Segmentation-
- By Deployment Mode Insights (Revenue, US$ Bn, 2020 - 2032)
- Cloud-based
- On-premises
- By Functionality Insights (Revenue, US$ Bn, 2020 - 2032)
- Document Management
- Workflow Management
- Reporting and Analytics
- Others
- By End User Insights (Revenue, US$ Bn, 2020 - 2032)
- Pharmaceutical Companies
- Biotechnology Companies
- Contract Research Organizations (CROs)
- Academic Research Institutions
- Regional Insights (Revenue, US$ Bn, 2020 - 2032)
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- South Africa
- North Africa
- Central Africa
- Key Players Insights
- Veeva Systems Inc.
- Medidata Solutions, Inc.
- Oracle Corporation
- Parexel International Corporation
- IBM Watson Health
- DrugDev (now part of Veeva)
- MasterControl, Inc.
- ArisGlobal LLC
- Dassault Systemes
- Trial Interactive
- Signant Health
- Forte Research Systems, Inc.
- Axiom Real-Time Metrics
- eClinical Solutions, LLC
- Bioclinica, Inc.
Table of Contents
1. Research Objectives and Assumptions
- Research Objectives
- Assumptions
- Abbreviations
2. Market Purview
- Report Description
- Market Definition and Scope
- Executive Summary
- Market Snippet, By Deployment Mode
- Market Snippet, By Functionality
- Market Snippet, By End User
- Market Snippet, By Region
3. Market Dynamics, Regulations, and Trends Analysis
- Market Dynamics
- Drivers
- Restraints
- Market Opportunities
- Regulatory Scenario
- Industry Trend
- Merger and Acquisitions
- New System Launches/Approvals
- Impact of COVID-19 Pandemic
4. Global Electronic Trial Master File (eTMF) Market, By Deployment Mode, 2020-2032, (US$ Bn)
- Introduction
- Market Share Analysis, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, 2021-2032
- Segment Trends
- Cloud-based
- Introduction
- Market Size and Forecast, 2020-2032, (US$ Bn)
- On-premises
- Introduction
- Market Size and Forecast, 2020-2032, (US$ Bn)
5. Global Electronic Trial Master File (eTMF) Market, By Functionality, 2020-2032, (US$ Bn)
- Introduction
- Market Share Analysis, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, 2021-2032
- Segment Trends
- Document Management
- Introduction
- Market Size and Forecast, 2020-2032, (US$ Bn)
- Workflow Management
- Introduction
- Market Size and Forecast, 2020-2032, (US$ Bn)
- Reporting and Analytics
- Introduction
- Market Size and Forecast, 2020-2032, (US$ Bn)
- Others
- Introduction
- Market Size and Forecast, 2020-2032, (US$ Bn)
6. Global Electronic Trial Master File (eTMF) Market, By End User, 2020-2032, (US$ Bn)
- Introduction
- Market Share Analysis, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, 2021-2032
- Segment Trends
- Pharmaceutical Companies
- Introduction
- Market Size and Forecast, 2020-2032, (US$ Bn)
- Biotechnology Companies
- Introduction
- Market Size and Forecast, 2020-2032, (US$ Bn)
- Contract Research Organizations (CROs)
- Introduction
- Market Size and Forecast, 2020-2032, (US$ Bn)
- Academic Research Institutions
- Introduction
- Market Size and Forecast, 2020-2032, (US$ Bn)
7. Global Electronic Trial Master File (eTMF) Market, By Region, 2020-2032, (US$ Bn)
- Introduction
- Market Share Analysis, By Region, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, 2021-2032
- North America
- Regional Trends
- Market Size and Forecast, By Deployment Mode, 2020-2032, (US$ Bn)
- Market Size and Forecast, By Functionality, 2020-2032, (US$ Bn)
- Market Size and Forecast, By End User, 2020-2032, (US$ Bn)
- Market Size and Forecast, By Country, 2020-2032, (US$ Bn)
- U.S.
- Canada
- Europe
- Regional Trends
- Market Size and Forecast, By Deployment Mode, 2020-2032, (US$ Bn)
- Market Size and Forecast, By Functionality, 2020-2032, (US$ Bn)
- Market Size and Forecast, By End User, 2020-2032, (US$ Bn)
- Market Size and Forecast, By Country, 2020-2032, (US$ Bn)
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- Regional Trends
- Market Size and Forecast, By Deployment Mode, 2020-2032, (US$ Bn)
- Market Size and Forecast, By Functionality, 2020-2032, (US$ Bn)
- Market Size and Forecast, By End User, 2020-2032, (US$ Bn)
- Market Size and Forecast, By Country, 2020-2032, (US$ Bn)
- China
- India
- Japan
- ASEAN
- Australia
- South Korea
- Rest of Asia Pacific
- Latin America
- Regional Trends
- Market Size and Forecast, By Deployment Mode, 2020-2032, (US$ Bn)
- Market Size and Forecast, By Functionality, 2020-2032, (US$ Bn)
- Market Size and Forecast, By End User, 2020-2032, (US$ Bn)
- Market Size and Forecast, By Country, 2020-2032, (US$ Bn)
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Middle East
- Regional Trends
- Market Size and Forecast, By Deployment Mode, 2020-2032, (US$ Bn)
- Market Size and Forecast, By Functionality, 2020-2032, (US$ Bn)
- Market Size and Forecast, By End User, 2020-2032, (US$ Bn)
- Market Size and Forecast, By Country, 2020-2032, (US$ Bn)
- Israel
- GCC Countries
- Rest of the Middle East
- Africa
- Regional Trends
- Market Size and Forecast, By Deployment Mode, 2020-2032, (US$ Bn)
- Market Size and Forecast, By Functionality, 2020-2032, (US$ Bn)
- Market Size and Forecast, By End User, 2020-2032, (US$ Bn)
- Market Size and Forecast, By Country/Region, 2020-2032, (US$ Bn)
- South Africa
- North Africa
- Central Africa
8. Competitive Landscape
- Company Profiles
- Veeva Systems Inc.
- Company Overview
- Product Portfolio
- Financial Performance
- Key Strategies
- Recent Developments/Updates
- Medidata Solutions, Inc.
- Company Overview
- Product Portfolio
- Financial Performance
- Key Strategies
- Recent Developments/Updates
- Oracle Corporation
- Company Overview
- Product Portfolio
- Financial Performance
- Key Strategies
- Recent Developments/Updates
- Parexel International Corporation
- Company Overview
- Product Portfolio
- Financial Performance
- Key Strategies
- Recent Developments/Updates
- IBM Watson Health
- Company Overview
- Product Portfolio
- Financial Performance
- Key Strategies
- Recent Developments/Updates
- DrugDev (now part of Veeva)
- Company Overview
- Product Portfolio
- Financial Performance
- Key Strategies
- Recent Developments/Updates
- MasterControl, Inc.
- Company Overview
- Product Portfolio
- Financial Performance
- Key Strategies
- Recent Developments/Updates
- ArisGlobal LLC
- Company Overview
- Product Portfolio
- Financial Performance
- Key Strategies
- Recent Developments/Updates
- Dassault Systemes
- Company Overview
- Product Portfolio
- Financial Performance
- Key Strategies
- Recent Developments/Updates
- Trial Interactive
- Company Overview
- Product Portfolio
- Financial Performance
- Key Strategies
- Recent Developments/Updates
- Signant Health
- Company Overview
- Product Portfolio
- Financial Performance
- Key Strategies
- Recent Developments/Updates
- Forte Research Systems, Inc.
- Company Overview
- Product Portfolio
- Financial Performance
- Key Strategies
- Recent Developments/Updates
- Axiom Real-Time Metrics
- Company Overview
- Product Portfolio
- Financial Performance
- Key Strategies
- Recent Developments/Updates
- eClinical Solutions, LLC
- Company Overview
- Product Portfolio
- Financial Performance
- Key Strategies
- Recent Developments/Updates
- Bioclinica, Inc.
- Company Overview
- Product Portfolio
- Financial Performance
- Key Strategies
- Recent Developments/Updates
- Veeva Systems Inc.
9. Analyst Recommendations
- Wheel of Fortune
- Analyst View
- Coherent Opportunity Map
10. Research Methodology
- References
- Research Methodology
- About us and Sales Contact









