 |
市场调查报告书
商品编码
1708601
全球肾上腺素自动注射器市场按类型、最终用户、分销管道和地区划分Global Epinephrine Autoinjector Market, By Type, By End User, By Distribution Channel, By Geography |
||||||
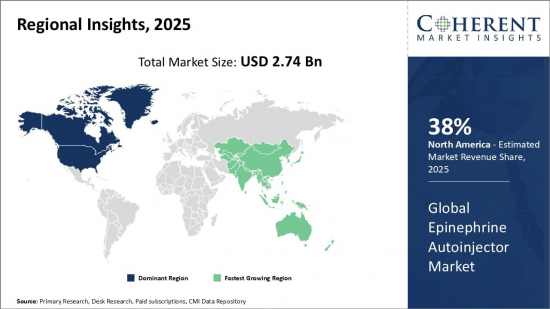
2025 年全球肾上腺素自动注射器市场规模估计为 27.4 亿美元,预计到 2032 年将达到 49.5 亿美元,2025 年至 2032 年的复合年增长率为 8.8%。
| 报告范围 | 报告详细信息 | ||
|---|---|---|---|
| 基准年 | 2024 | 2025年的市场规模 | 27.4亿美元 |
| 效能数据 | 2020-2024 | 预测期 | 2025-2032 |
| 预测期间:2025年至2032年的复合年增长率 | 8.80% | 2032年价值预测 | 49.5亿美元 |
肾上腺素自动注射器是一种一次性自动注射器,用于过敏反应(包括过敏性休克)的紧急治疗。过敏性休克是一种危及生命的疾病,由对某些食物、药物、昆虫叮咬或其他过敏原的极度过敏反应引起。过敏性休克的症状包括低血压、口腔和喉咙肿胀、皮疹、呼吸困难和休克。发生过敏反应时,应及时注射肾上腺素以减缓症状的进展。与注射器相比,肾上腺素自动注射器可以快速自动注射肾上腺素,从而降低伤亡的风险。
市场动态
全球肾上腺素自动注射器市场的成长是由全球过敏反应盛行率的上升所推动的。根据食物过敏和过敏反应网路 (FAAN) 发布的数据,仅在美国每年就会发生 50,000 例过敏反应病例,其中 150 至 200 例导致死亡。人们对过敏相关危及生命的疾病的认识不断提高,以及先进的肾上腺素自动注射器的普及,正在推动市场需求。然而,这些设备的成本以及新兴经济体对替代治疗方案的偏好限制了市场的成长。创新肾上腺素自动注射器的开发预计在未来几年为製造商带来丰厚的机会,其易用性、准确性和应用范围均有所提高。
由于技术进步、人们对过敏反应的认识不断提高以及对使用者友善设计的日益重视,肾上腺素自动注射器市场正在出现值得关注的趋势。无针替代品和用于培训的数位健康平台正成为人们关注的焦点,提高了患者的舒适度和可及性。市场也在应对疫情后不断变化的情况,需要医疗保健系统和供应链具有适应性。持续的技术创新、改进的设备功能和策略联盟正在进一步塑造肾上腺素自动注射器市场动态,旨在满足快速变化的医疗保健环境中高风险个体和医疗保健提供者的需求。
研究的主要特点
本报告对全球肾上腺素自动注射器市场进行了详细分析,并以 2024 年为基准年,展示了预测期(2025-2032 年)的市场规模和年复合成长率(CAGR%)。
它还强调了各个领域的潜在商机,并说明了该市场的有吸引力的投资提案矩阵。
它还提供了有关市场驱动因素、限制因素、机会、新产品发布和核准、市场趋势、区域前景和主要企业采用的竞争策略的重要见解。
全球肾上腺素自动注射器市场的主要企业根据公司亮点、产品系列、关键亮点、性能和策略等参数进行分析。
主要企业包括 Mylan、Teva Pharmaceutical、Impax Laboratories、Adamis Pharmaceuticals Corporation、辉瑞、ALK Abello、Lincoln Medical、Hospira、Sanofi 和 Kaleo。
从本报告中获得的见解将使负责人和企业经营团队能够就未来的产品发布、新兴趋势、市场扩张和行销策略做出明智的决策。
全球肾上腺素自动注射器市场报告迎合了该行业的各个相关人员,例如投资者、供应商、产品製造商、经销商、新进业者和金融分析师。
透过分析全球肾上腺素自动注射器市场所使用的各种策略矩阵,相关人员可以更轻鬆地做出决策。
目录
第一章 调查目的与前提条件
- 研究目标
- 先决条件
- 简称
第二章 市场展望
- 报告描述
- 市场定义和范围
- 执行摘要
- 一致的机会图(COM)
第三章市场动态、法规与趋势分析
- 市场动态
- 影响分析
- 监管情景
- 服务产品组合
- PEST分析
- 波特分析
- 併购场景
- 管道分析
4. 全球肾上腺素自动注射器市场-冠状病毒(COVID-19)大流行的影响
- COVID-19流行病学
- 供需侧分析
- 经济影响
5. 2020 年至 2032 年全球肾上腺素自动注射器市场(按类型)
- 介绍
- 15mg
- 3mg
- 5mg
6. 2020 年至 2032 年全球肾上腺素自动注射器市场(按最终用户划分)
- 介绍
- 医院
- 诊所
- 个人客户
7. 全球肾上腺素自动注射器市场(依通路划分),2020-2032 年
- 介绍
- 零售药局
- 医院药房
- 网路药局
8. 2020 年至 2032 年全球肾上腺素自动注射器市场(按地区)
- 介绍
- 北美洲
- 欧洲
- 亚太地区
- 拉丁美洲
- 中东
- 非洲
第九章 竞争态势
- Mylan
- Teva Pharmaceutical
- Impax Laboratories
- Adamis Pharmaceuticals Corporation
- Pfizer
- ALK Abello
- Lincoln Medical
- Hospira
- Sanofi
- Kaleo
第 10 章 章节
- 调查方法
- 关于出版商
Global Epinephrine Autoinjector Market is estimated to be valued at USD 2.74 Bn in 2025 and is expected to reach USD 4.95 Bn by 2032, growing at a compound annual growth rate (CAGR) of 8.8% from 2025 to 2032.
| Report Coverage | Report Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 2.74 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 8.80% | 2032 Value Projection: | USD 4.95 Bn |

Epinephrine autoinjector is a single-use disposable automatic injection device that is intended for emergency treatment of allergic reactions including anaphylaxis. Anaphylaxis is a life-threatening condition that occur due to an extreme allergic reaction to certain foods, medicines, insects bites and other allergens. The symptoms of anaphylaxis include low blood pressure, swelling of the mouth or throat, rashes, breathing difficulty and shock. In case of anaphylaxis, epinephrine needs to be administered in a timely manner to curb the advancement of symptoms. Epinephrine autoinjectors enable rapid self-administration of epinephrine as compared to syringes, thereby, reducing the risk of casualty.
Market Dynamics:
Global epinephrine autoinjector market growth is driven by rising prevalence of anaphylaxis worldwide. According to the data published by FAAN (The Food Allergy and Anaphylaxis Network), 50,000 cases of anaphylaxis occur annually in the U.S. alone, out of which 150-200 result in death. Increased awareness regarding life-threatening conditions associated with allergies along with availability of advanced epinephrine autoinjector devices are fueling the market demand. However, cost of these devices and preference for alternative treatment options in developing nations limits the market growth. Development of innovative epinephrine autoinjectors with improved usability, accuracy and larger coverage area is expected to present lucrative opportunities for manufacturers in the coming years.
The epinephrine autoinjector market is witnessing notable trends, driven by advancements in technology, increased awareness of anaphylaxis, and a growing emphasis on user-friendly designs. Needle-free alternatives and digital health platforms for training are gaining prominence, enhancing patient comfort and accessibility. The market is also responding to the evolving landscape influenced by the pandemic, necessitating adaptability in healthcare systems and supply chains. Continuous innovation, improved device features, and strategic collaborations are further shaping the dynamics of the epinephrine autoinjector market, aiming to address the needs of at-risk individuals and healthcare providers in a rapidly changing healthcare environment.
Key features of the study:
This report provides in-depth analysis of the global epinephrine autoinjector market, and provides market size (US$ Bn) and compound annual growth rate (CAGR%) for the forecast period (2025-2032), considering 2024 as the base year
It elucidates potential revenue opportunities across different segments and explains attractive investment proposition matrices for this market
This study also provides key insights about market drivers, restraints, opportunities, new product launches or approval, market trends, regional outlook, and competitive strategies adopted by key players
It profiles key players in the global epinephrine autoinjector market based on the following parameters - company highlights, products portfolio, key highlights, financial performance, and strategies
Key companies covered as a part of this study include Mylan, Teva Pharmaceutical, Impax Laboratories, Adamis Pharmaceuticals Corporation, Pfizer, ALK Abello, Lincoln Medical, Hospira, Sanofi, and Kaleo
Insights from this report would allow marketers and the management authorities of the companies to make informed decisions regarding their future product launches, type up-gradation, market expansion, and marketing tactics
Global epinephrine autoinjector market report caters to various stakeholders in this industry including investors, suppliers, product manufacturers, distributors, new entrants, and financial analysts
Stakeholders would have ease in decision-making through various strategy matrices used in analyzing the global epinephrine autoinjector market
Detailed Segmentation:
- Global Epinephrine Autoinjector Market, By Type
- 15mg
- 3mg
- 5mg
- Global Epinephrine Autoinjector Market, By End-User
- Hospitals
- Clinics
- Individual Customers
- Global Epinephrine Autoinjector Market, By Distribution Channel
- Retail Pharmacies
- Hospital Pharmacies
- Online Pharmacies
- Global Epinephrine Autoinjector Market, By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East
- Africa
- Company Profiles
- Mylan
- Teva Pharmaceutical
- Impax Laboratories
- Adamis Pharmaceuticals Corporation
- Pfizer
- ALK Abello
- Lincoln Medical
- Hospira
- Sanofi
- kaleo
Table of Contents
1. Research Objectives and Assumptions
- Research Objectives
- Assumptions
- Abbreviations
2. Market Purview
- Report Description
- Market Definition and Scope
- Executive Summary
- Global Epinephrine Autoinjector Market, By Type
- Global Epinephrine Autoinjector Market, By End User
- Global Epinephrine Autoinjector Market, By Distribution Channel
- Global Epinephrine Autoinjector Market, By Region
- Coherent Opportunity Map (COM)
3. Market Dynamics, Regulations, and Trends Analysis
- Market Dynamics
- Growing prevalence of anaphylaxis
- Rising Prevalence of Food Allergies
- Impact Analysis
- Key Highlights
- Regulatory Scenario
- Service offering Portfolio
- PEST Analysis
- PORTER's Analysis
- Merger and Acquisition Scenario
- Pipeline Analysis
4. Global Epinephrine Autoinjector Market- Impact of Coronavirus (COVID-19) Pandemic
- COVID-19 Epidemiology
- Supply Side and Demand Side Analysis
- Economic Impact
5. Global Epinephrine Autoinjector Market, By Type, 2020-2032, (US$ Bn)
- Introduction
- Market Share Analysis, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, 2021 - 2032
- Segment Trends
- 0.15mg
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (US$ Bn)
- 0.3mg
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (US$ Bn)
- 0.5mg
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (US$ Bn)
6. Global Epinephrine Autoinjector Market, By End User, 2020-2032, (US$ Bn)
- Introduction
- Market Share Analysis, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, 2021 - 2032
- Segment Trends
- Hospitals
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (US$ Bn)
- Clinics
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (US$ Bn)
- Individual Customers
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (US$ Bn)
7. Global Epinephrine Autoinjector Market, By Distribution Channel, 2020-2032, (US$ Bn)
- Introduction
- Market Share Analysis, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, 2021 - 2032
- Segment Trends
- Retail Pharmacies
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (US$ Bn)
- Hospital Pharmacies
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (US$ Bn)
- Online Pharmacies
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (US$ Bn)
8. Global Epinephrine Autoinjector Market, By Region, 2020-2032, (US$ Bn)
- Introduction
- Market Share Analysis, By Region, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, For Region, 2021 -2032
- Country Trends
- North America
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, By Type, 2020-2032, (US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By End User, 2020-2032, (US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Distribution Channel, 2020-2032, (US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Country, 2020-2032, (US$ Bn)
- U.S.
- Canada
- Europe
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, By Type, 2020-2032, (US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By End User, 2020-2032, (US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Distribution Channel, 2020-2032, (US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Country, 2020-2032, (US$ Bn)
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, By Type, 2020-2032, (US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By End User, 2020-2032, (US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Distribution Channel, 2020-2032, (US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Country, 2020-2032, (US$ Bn)
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Latin America
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, By Type, 2020-2032, (US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By End User, 2020-2032, (US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Distribution Channel, 2020-2032, (US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Country, 2020-2032, (US$ Bn)
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Middle East
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, By Type, 2020-2032, (US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By End User, 2020-2032, (US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Distribution Channel, 2020-2032, (US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Country, 2020-2032, (US$ Bn)
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, By Type, 2020-2032, (US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By End User, 2020-2032, (US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Distribution Channel, 2020-2032, (US$ Bn)
- Market Size and Forecast, and Y-o-Y Growth, By Country/Region, 2020-2032, (US$ Bn)
- North Africa
- Central Africa
- South Africa
9. Competitive Landscape
- Mylan
- Company Highlights
- Type Portfolio
- Key Highlights
- Financial Performance
- Strategies
- Teva Pharmaceutical
- Company Highlights
- Type Portfolio
- Key Highlights
- Financial Performance
- Strategies
- Impax Laboratories
- Company Highlights
- Type Portfolio
- Key Highlights
- Financial Performance
- Strategies
- Adamis Pharmaceuticals Corporation
- Company Highlights
- Type Portfolio
- Key Highlights
- Financial Performance
- Strategies
- Pfizer
- Company Highlights
- Type Portfolio
- Key Highlights
- Financial Performance
- Strategies
- ALK Abello
- Company Highlights
- Type Portfolio
- Key Highlights
- Financial Performance
- Strategies
- Lincoln Medical
- Company Highlights
- Type Portfolio
- Key Highlights
- Financial Performance
- Strategies
- Hospira
- Company Highlights
- Type Portfolio
- Key Highlights
- Financial Performance
- Strategies
- Sanofi
- Company Highlights
- Type Portfolio
- Key Highlights
- Financial Performance
- Strategies
- Kaleo
- Company Highlights
- Type Portfolio
- Key Highlights
- Financial Performance
- Strategies
- Analyst Views
10. Section
- Research Methodology
- About us








