 |
市场调查报告书
商品编码
1665063
基于肿瘤的体内 CRO 市场机会、成长动力、产业趋势分析与预测 2025 - 2034Oncology Based In-vivo CRO Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
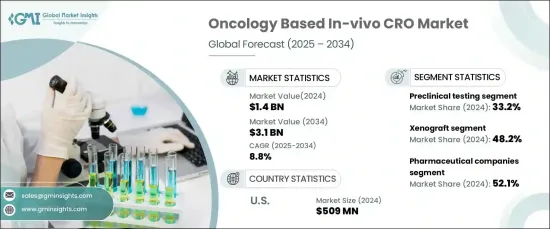
2024 年,全球基于肿瘤的体内 CRO 市场规模达到 14 亿美元,预计 2025 年至 2034 年期间将以 8.8% 的强劲年复合成长率(CAGR) 增长。 这一显着的市场扩张是由对免疫肿瘤疗法的需求不断增长、体内模型的不断进步。
小型生物技术和製药公司在推动肿瘤药物发展方面发挥着至关重要的作用,从而加速了整体市场的成长。这些公司专注于创新治疗方法,包括基因疗法和基于抗体的药物,旨在解决肿瘤学领域未满足的医疗需求。他们在癌症研究方面的专业方法继续推动对临床前和体内测试服务的需求,进一步促进市场上升趋势。
| 市场范围 | |
|---|---|
| 起始年份 | 2024 |
| 预测年份 | 2025-2034 |
| 起始值 | 14亿美元 |
| 预测值 | 31亿美元 |
| 复合年增长率 | 8.8% |
肿瘤体内 CRO 市场按服务类型细分,主要类别包括临床前测试、功效测试、毒理学研究、药物动力学等。 2024年临床前测试占据最大的市场份额,为33.2%。这种主导地位是由于临床前测试在药物开发中发挥的至关重要的作用,确保新疗法在进入人体临床试验之前的安全性和有效性。美国 FDA 和 EMA 等监管机构要求临床前测试是临床试验开始前的关键步骤。这些研究提供了有关药物毒性、药效学和治疗效果的重要资料,使其成为肿瘤学研究过程中不可或缺的一部分。
市场也按模型类型细分,包括异种移植、同基因和其他模型。异种移植模型在 2024 年占据市场主导地位,占有 48.2% 的份额。这些模型因其能够紧密模拟人类肿瘤生物学而受到广泛青睐,为抗癌药物的有效性提供了关键的见解。异种移植模型是将人类肿瘤组织植入免疫功能低下的小鼠体内,这可以准确模拟人类癌症的进展并增强药物疗效的评估。
在美国,肿瘤体内 CRO 市场在 2024 年的规模为 5.09 亿美元,占据区域主导地位。该国的市场领导地位可归因于对肿瘤学研究的大量投资以及其製药和生物技术领域的实力。由于许多公司专注于肿瘤药物开发,美国严重依赖将临床前和体内研究外包给 CRO。这一成长趋势支持了市场的持续扩张,这得益于肿瘤治疗的创新和对体内测试解决方案不断增长的需求。
目录
第 1 章:方法论与范围
- 市场范围和定义
- 研究设计
- 研究方法
- 资料收集方法
- 基础估算与计算
- 基准年计算
- 市场估计的主要趋势
- 预测模型
- 初步研究和验证
- 主要来源
- 资料探勘来源
第 2 章:执行摘要
第 3 章:产业洞察
- 产业生态系统分析
- 产业衝击力
- 成长动力
- 免疫肿瘤疗法的需求不断增长
- 体内模型的进展
- 精准肿瘤学投资不断增加
- 小型生物技术和製药公司批准的肿瘤药物数量不断增加
- 产业陷阱与挑战
- 肿瘤药物审批的严格监管要求
- 存在强有力的替代方案
- 成长动力
- 成长潜力分析
- 监管格局
- 技术格局
- 专利分析
- 未来市场趋势
- 波特的分析
- PESTEL 分析
第四章:竞争格局
- 介绍
- 公司市占率分析
- 主要市场参与者的竞争分析
- 竞争定位矩阵
- 策略展望
第 5 章:市场估计与预测:按服务,2021 年至 2034 年
- 主要趋势
- 临床前测试
- 功效测试
- 毒理学研究
- 药物动力学
- 其他服务
第六章:市场估计与预测:依模型,2021 – 2034 年
- 主要趋势
- 异种移植
- 患者来源的异种移植
- 细胞系来源的异种移植
- 其他异种移植
- 同源
- 其他型号
第 7 章:市场估计与预测:依最终用途,2021 年至 2034 年
- 主要趋势
- 製药公司
- 生技公司
- 其他最终用户
第 8 章:市场估计与预测:按地区,2021 年至 2034 年
- 主要趋势
- 北美洲
- 我们
- 加拿大
- 欧洲
- 德国
- 英国
- 法国
- 西班牙
- 义大利
- 亚太地区
- 中国
- 日本
- 印度
- 澳洲
- 韩国
- 拉丁美洲
- 巴西
- 墨西哥
- 阿根廷
- 中东和非洲
- 南非
- 沙乌地阿拉伯
- 阿联酋
第九章:公司简介
- Charles River Laboratories
- Crown Bioscience
- Eurofins Scientific
- Evotec SE
- ICON plc
- IMV
- Laboratory Corporation of America Holdings
- Medidata
- Merck KGaA
- OncoOne
- Pharmaron
- Taconic Biosciences
- The Jackson Laboratory
- Thermo Fisher Scientific
- WuXi AppTec
The Global Oncology Based In-Vivo CRO Market reached USD 1.4 billion in 2024 and is expected to grow at a robust compound annual growth rate (CAGR) of 8.8% from 2025 to 2034. This significant market expansion is being driven by the increasing demand for immuno-oncology therapies, ongoing advancements in in-vivo models, and a rise in oncology drug approvals from both biotechnology and pharmaceutical companies.

Small biotechnology and pharmaceutical companies are playing a crucial role in driving the development of oncology drugs, thus accelerating overall market growth. These companies focus on innovative treatments, including gene therapies and antibody-based drugs, which are designed to address unmet medical needs in the oncology space. Their specialized approach to cancer research continues to fuel the demand for preclinical and in-vivo testing services, further contributing to the market's upward trajectory.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $1.4 Billion |
| Forecast Value | $3.1 Billion |
| CAGR | 8.8% |
The oncology in-vivo CRO market is segmented by service type, with the primary categories being preclinical testing, efficacy testing, toxicology studies, pharmacokinetics, and others. Preclinical testing held the largest share of the market in 2024, accounting for 33.2%. This dominance is due to the vital role preclinical testing plays in drug development, ensuring the safety and efficacy of new therapies before they progress to human clinical trials. Regulatory authorities such as the U.S. FDA and EMA require preclinical testing as a critical step before clinical trials can commence. These studies provide essential data on drug toxicity, pharmacodynamics, and therapeutic effects, making them indispensable in the oncology research pipeline.
The market is also segmented by model type, including xenograft, syngeneic, and other models. Xenograft models led the market in 2024, holding a 48.2% share. These models are widely preferred due to their ability to closely mimic human tumor biology, offering crucial insights into the effectiveness of cancer drugs. Xenograft models involve implanting human tumor tissues into immunocompromised mice, which enables an accurate simulation of human cancer progression and enhances the evaluation of drug efficacy.
In the United States, the oncology in-vivo CRO market accounted for USD 509 million in 2024, dominating the regional landscape. The country's market leadership can be attributed to significant investments in oncology research and the strength of its pharmaceutical and biotechnology sectors. With numerous companies focused on oncology drug development, the U.S. heavily relies on outsourcing preclinical and in-vivo studies to CROs. This growing trend supports continued market expansion, driven by innovations in oncology therapies and a rising demand for in-vivo testing solutions.
Table of Contents
Chapter 1 Methodology & Scope
- 1.1 Market scope & definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Base estimates & calculations
- 1.3.1 Base year calculation
- 1.3.2 Key trends for market estimation
- 1.4 Forecast model
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.5.2 Data mining sources
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Growing demand for immuno-oncology therapies
- 3.2.1.2 Advancements in in-vivo models
- 3.2.1.3 Growing investments in precision oncology
- 3.2.1.4 Growing oncology drug approvals from small biotechnology and pharmaceutical companies
- 3.2.2 Industry pitfalls & challenges
- 3.2.2.1 Stringent regulatory requirements for oncology drug approvals
- 3.2.2.2 Presence of strong alternatives
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.5 Technological landscape
- 3.6 Patent Analysis
- 3.7 Future market trends
- 3.8 Porter’s analysis
- 3.9 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.3 Competitive analysis of major market players
- 4.4 Competitive positioning matrix
- 4.5 Strategy outlook
Chapter 5 Market Estimates and Forecast, By Service, 2021 – 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Preclinical testing
- 5.3 Efficacy testing
- 5.4 Toxicology studies
- 5.5 Pharmacokinetics
- 5.6 Other services
Chapter 6 Market Estimates and Forecast, By Model, 2021 – 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Xenograft
- 6.2.1 Patient derived xenograft
- 6.2.2 Cell-line derived xenograft
- 6.2.3 Other xenografts
- 6.3 Syngeneic
- 6.4 Other models
Chapter 7 Market Estimates and Forecast, By End Use, 2021 – 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Pharmaceutical companies
- 7.3 Biotechnology companies
- 7.4 Other end users
Chapter 8 Market Estimates and Forecast, By Region, 2021 – 2034 ($ Mn)
- 8.1 Key trends
- 8.2 North America
- 8.2.1 U.S.
- 8.2.2 Canada
- 8.3 Europe
- 8.3.1 Germany
- 8.3.2 UK
- 8.3.3 France
- 8.3.4 Spain
- 8.3.5 Italy
- 8.4 Asia Pacific
- 8.4.1 China
- 8.4.2 Japan
- 8.4.3 India
- 8.4.4 Australia
- 8.4.5 South Korea
- 8.5 Latin America
- 8.5.1 Brazil
- 8.5.2 Mexico
- 8.5.3 Argentina
- 8.6 Middle East and Africa
- 8.6.1 South Africa
- 8.6.2 Saudi Arabia
- 8.6.3 UAE
Chapter 9 Company Profiles
- 9.1 Charles River Laboratories
- 9.2 Crown Bioscience
- 9.3 Eurofins Scientific
- 9.4 Evotec SE
- 9.5 ICON plc
- 9.6 IMV
- 9.7 Laboratory Corporation of America Holdings
- 9.8 Medidata
- 9.9 Merck KGaA
- 9.10 OncoOne
- 9.11 Pharmaron
- 9.12 Taconic Biosciences
- 9.13 The Jackson Laboratory
- 9.14 Thermo Fisher Scientific
- 9.15 WuXi AppTec









