 |
市场调查报告书
商品编码
1684639
药品稳定性与储存服务市场机会、成长动力、产业趋势分析与预测 2025 - 2034Pharmaceutical Stability and Storage Services Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
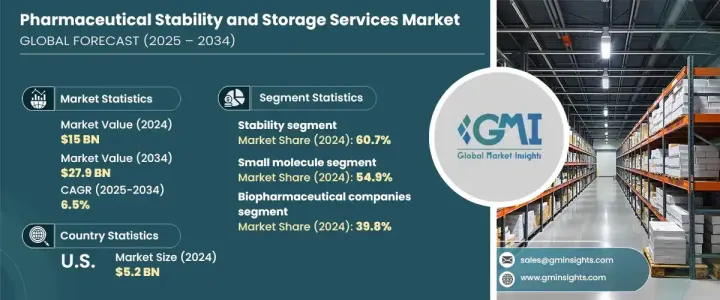
2024 年全球药品稳定性和储存服务市场价值为 150 亿美元,预计 2025 年至 2034 年的复合年增长率为 6.5%。製药公司面临巨大的压力,需要确保其产品符合严格的品质标准并在整个保质期内保持安全有效。这大大增加了对稳定性和储存服务的需求,这些服务对于在特定条件下维持药物、生物製剂和医疗器材的功效至关重要。此外,随着企业寻求具有成本效益和专业的解决方案来满足监管和营运需求,将製药流程外包给专业服务提供者的趋势日益兴起,进一步推动了市场成长。
药品稳定性和储存服务包括专门的解决方案,确保产品长期保持其预期的安全性、有效性和品质。这些服务包括在特定环境条件下进行控制储存和系统稳定性测试,以评估药物在整个生命週期内的行为。美国食品药物管理局 (FDA) 和欧洲药品管理局 (EMA) 等监管机构要求进行严格的稳定性测试以确保合规性,从而推动全球对这些服务的需求。
| 市场范围 | |
|---|---|
| 起始年份 | 2024 |
| 预测年份 | 2025-2034 |
| 起始值 | 150亿美元 |
| 预测值 | 279亿美元 |
| 复合年增长率 | 6.5% |
按服务类型细分的市场包括稳定性服务和储存服务。在稳定性类别中,药物物质稳定性、稳定性指示方法验证、加速稳定性测试、光稳定性测试和其他测试方法等服务占主导地位,在 2024 年占据 60.7% 的市场份额。同时,储存部分分为冷藏和非冷藏解决方案,以满足医药产品的不同需求。
根据分子类型,市场分为小分子和大分子,分别进一步细分为商业产品和研究产品。小分子药物在 2024 年将占据 54.9% 的市场份额,仍然是製药业的基石。它们在口服药物中的广泛应用、成熟的製造流程、成本效益和可扩展性使它们备受青睐。这些因素推动了对稳定性和根据其特定需求量身定制的储存服务的持续需求。
在美国,药品稳定性和储存服务市场在 2024 年创造了 52 亿美元的收入,成为全球收入的主要贡献者。随着生物技术的进步,对生物製剂的需求不断增长,对冷藏和冷冻储存等专业储存解决方案的需求也激增。美国作为生物製剂和生物相似药生产的领导者,严重依赖加速和光稳定性测试等服务来维持这些高价值产品的品质和安全。
目录
第 1 章:方法论与范围
第 2 章:执行摘要
第 3 章:产业洞察
- 产业生态系统分析
- 产业衝击力
- 成长动力
- 越来越关注监理合规性
- 增加对药物开发和研究的投资
- 提高药品稳定性和储存的技术创新
- 全球供应链的扩张
- 产业陷阱与挑战
- 专用储存解决方案成本高昂
- 与运输和物流相关的担忧
- 成长动力
- 成长潜力分析
- 监管格局
- 技术格局
- 未来市场趋势
- 差距分析
- 波特的分析
- PESTEL 分析
第四章:竞争格局
- 介绍
- 主要市场参与者的竞争分析
- 竞争定位矩阵
- 战略展望
第 5 章:市场估计与预测:按服务类型,2021 年至 2034 年
- 主要趋势
- 稳定
- 药物物质
- 稳定性指示方法验证
- 加速稳定性测试
- 光稳定性测试
- 其他稳定性测试方法
- 贮存
- 寒冷的
- 冰冻
- 冷藏
- 受控
- 低温
- 非冷
- 寒冷的
第 6 章:市场估计与预测:按分子类型,2021 – 2034 年
- 主要趋势
- 小分子
- 商业产品
- 研究产品
- 大分子
- 商业产品
- 研究产品
第 7 章:市场估计与预测:依最终用途,2021 年至 2034 年
- 主要趋势
- 生物製药公司
- 合约製造组织
- 合约研究组织
- 其他最终用户
第 8 章:市场估计与预测:按地区,2021 年至 2034 年
- 主要趋势
- 北美洲
- 我们
- 加拿大
- 欧洲
- 德国
- 英国
- 法国
- 西班牙
- 义大利
- 亚太地区
- 中国
- 日本
- 印度
- 澳洲
- 韩国
- 拉丁美洲
- 巴西
- 墨西哥
- 阿根廷
- 中东和非洲
- 南非
- 沙乌地阿拉伯
- 阿联酋
第九章:公司简介
- Alcami Corporation
- Almac Group
- Auriga Research
- Catalent
- Charles River Laboratories
- Element Materials Technology
- Eurofins Scientific
- Intertek Group
- Lucideon
- PD Partners
- Precision Stability Storage
- Q Laboratories
- Q1 Scientific
- Reading Scientific Services
- Roylance Stability Storage
The Global Pharmaceutical Stability And Storage Services Market was valued at USD 15 billion in 2024 and is forecasted to grow at a CAGR of 6.5% from 2025 to 2034. The market is experiencing robust growth due to increasing emphasis on regulatory compliance, escalating investments in drug development and research, and the expanding global supply chains that necessitate specialized solutions. Pharmaceutical companies are under immense pressure to ensure their products meet stringent quality standards and remain safe and effective throughout their shelf life. This has significantly boosted demand for stability and storage services, which are integral to maintaining the efficacy of drugs, biologics, and medical devices under specified conditions. Additionally, the rising trend of outsourcing pharmaceutical processes to specialized service providers is further propelling market growth as companies seek cost-efficient and expert solutions to meet regulatory and operational demands.

Pharmaceutical stability and storage services encompass specialized solutions that ensure products retain their intended safety, efficacy, and quality over time. These services include controlled storage under specified environmental conditions and systematic stability testing to assess the behavior of pharmaceutical products throughout their lifecycle. Regulatory agencies like the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) mandate rigorous stability testing to ensure compliance, driving demand for these services globally.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $15 Billion |
| Forecast Value | $27.9 Billion |
| CAGR | 6.5% |
The market, segmented by service type, includes stability and storage services. Within the stability category, services such as drug substance stability, stability-indicating method validation, accelerated stability testing, photostability testing, and other testing methods dominate, contributing to 60.7% of the market share in 2024. Stability testing is crucial for ensuring the safety and efficacy of drugs and is a requirement for both new drug applications and post-market surveillance. Meanwhile, the storage segment is divided into cold and non-cold storage solutions, catering to the varying needs of pharmaceutical products.
By molecule type, the market is categorized into small molecules and large molecules each further segmented into commercial products and research products. Small molecules, which accounted for 54.9% of the market share in 2024, remain a cornerstone of the pharmaceutical industry. Their widespread use in oral medications, established manufacturing processes, cost-effectiveness, and scalability make them highly desirable. These factors drive consistent demand for stability and storage services tailored to their specific requirements.
In the United States, the pharmaceutical stability and storage services market generated USD 5.2 billion in 2024, making it a key contributor to global revenue. The growing demand for biologics, driven by advancements in biotechnology, has created a surge in the need for specialized storage solutions such as refrigerated and frozen storage. The U.S., being a leader in the production of biologics and biosimilars, relies heavily on services like accelerated and photostability testing to maintain the quality and safety of these high-value products.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Base estimates and calculations
- 1.3.1 Base year calculation
- 1.3.2 Key trends for market estimation
- 1.4 Forecast model
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.5.2 Data mining sources
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Growing focus on regulatory compliance
- 3.2.1.2 Increasing investments in drug development and research
- 3.2.1.3 Technological innovations enhancing pharmaceutical stability and storage
- 3.2.1.4 Expansion in global supply chains
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 High cost associated with specialized storage solutions
- 3.2.2.2 Concerns related to transportation and logistics
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.5 Technological landscape
- 3.6 Future market trends
- 3.7 Gap analysis
- 3.8 Porter's analysis
- 3.9 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Competitive analysis of major market players
- 4.3 Competitive positioning matrix
- 4.4 Strategy outlook
Chapter 5 Market Estimates and Forecast, By Service Type, 2021 – 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Stability
- 5.2.1 Drug substance
- 5.2.2 Stability indicating method validation
- 5.2.3 Accelerated stability testing
- 5.2.4 Photostability testing
- 5.2.5 Other stability testing methods
- 5.3 Storage
- 5.3.1 Cold
- 5.3.1.1 Frozen
- 5.3.1.2 Refrigerated
- 5.3.1.3 Controlled
- 5.3.1.4 Cryogenic
- 5.3.2 Non-cold
- 5.3.1 Cold
Chapter 6 Market Estimates and Forecast, By Molecule Type, 2021 – 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Small molecule
- 6.2.1 Commercial products
- 6.2.2 Research products
- 6.3 Large molecule
- 6.3.1 Commercial products
- 6.3.2 Research products
Chapter 7 Market Estimates and Forecast, By End Use, 2021 – 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Biopharmaceutical companies
- 7.3 Contract manufacturing organization
- 7.4 Contract research organization
- 7.5 Other end users
Chapter 8 Market Estimates and Forecast, By Region, 2021 – 2034 ($ Mn)
- 8.1 Key trends
- 8.2 North America
- 8.2.1 U.S.
- 8.2.2 Canada
- 8.3 Europe
- 8.3.1 Germany
- 8.3.2 UK
- 8.3.3 France
- 8.3.4 Spain
- 8.3.5 Italy
- 8.4 Asia Pacific
- 8.4.1 China
- 8.4.2 Japan
- 8.4.3 India
- 8.4.4 Australia
- 8.4.5 South Korea
- 8.5 Latin America
- 8.5.1 Brazil
- 8.5.2 Mexico
- 8.5.3 Argentina
- 8.6 Middle East and Africa
- 8.6.1 South Africa
- 8.6.2 Saudi Arabia
- 8.6.3 UAE
Chapter 9 Company Profiles
- 9.1 Alcami Corporation
- 9.2 Almac Group
- 9.3 Auriga Research
- 9.4 Catalent
- 9.5 Charles River Laboratories
- 9.6 Element Materials Technology
- 9.7 Eurofins Scientific
- 9.8 Intertek Group
- 9.9 Lucideon
- 9.10 PD Partners
- 9.11 Precision Stability Storage
- 9.12 Q Laboratories
- 9.13 Q1 Scientific
- 9.14 Reading Scientific Services
- 9.15 Roylance Stability Storage








