 |
市场调查报告书
商品编码
1698228
异位性皮肤炎药物市场机会、成长动力、产业趋势分析及 2025 - 2034 年预测Atopic Dermatitis Drugs Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
2024 年全球异位性皮肤炎药物市场价值为 121 亿美元,预计 2025 年至 2034 年期间的复合年增长率为 9.9%。异位性皮肤炎药物有助于控制皮肤发炎、搔痒和干燥,针对病情发作并控制病情。这些治疗方法包括局部用药、全身性治疗和生物製剂,根据病情严重程度进行选择。异位性皮肤炎盛行率的上升、强大的药物管道以及研发投入的增加正在推动市场成长。製药公司正在推出新产品,积极的临床试验结果正在推动收入成长。
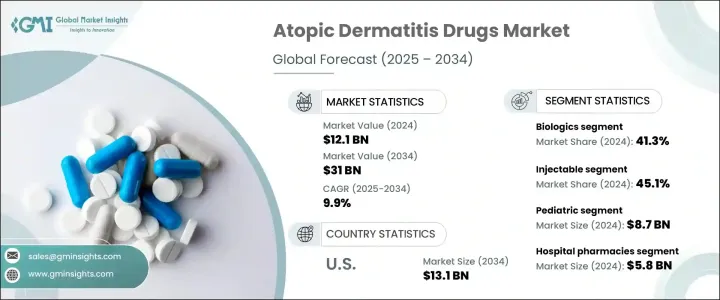
2023年,异位性皮肤炎药物市场价值为111亿美元。 2024 年,生物製剂领域将引领市场,占据 41.3% 的收入份额,这得益于更高的疗效和长期的症状控制。由于生物製剂具有优异的治疗效果和不断增长的产品审批,其需求持续上升。
| 市场范围 | |
|---|---|
| 起始年份 | 2024 |
| 预测年份 | 2025-2034 |
| 起始值 | 121亿美元 |
| 预测值 | 310亿美元 |
| 复合年增长率 | 9.9% |
根据给药途径,市场分为注射、外用和口服治疗。 2024 年註射剂市场占据主导地位,收入份额达 45.1%。针对发炎途径的新型生物注射剂,如 IL-4、IL-13 和 JAK 抑制剂,正在扩大治疗选择。监管部门的批准加速了产品的推出,提高了主要市场的可及性和采用率。
患者人口统计细分包括儿科和成人类别。 2024 年儿科领域收入最高,为 87 亿美元,这主要是由于儿童异位性皮肤炎发生率高。随着监管机构批准更多针对年轻患者的治疗方法,对儿科治疗的需求持续成长。专为儿科用途设计的生物製剂的快速审批进一步推动了市场扩张。
分销管道包括医院药房、零售药局和电子商务。 2024 年,医院药局的收入为 58 亿美元。这些药房在管理需要专门或系统治疗的患者方面发挥着至关重要的作用,确保对治疗结果和潜在副作用进行适当的监测。
美国市场经历了显着成长,营收从 2023 年的 47 亿美元成长到预计 2034 年的 131 亿美元。不断增强的认识和宣传活动正在增加研究资金,并改善患者获得先进治疗方案的机会。监管机构继续加快生物製剂的审批,特别是针对重症病例和儿科患者,进一步支持市场成长。
目录
第一章:方法论与范围
第二章:执行摘要
第三章:行业洞察
- 产业生态系统分析
- 产业衝击力
- 成长动力
- 异位性皮肤炎盛行率上升
- 生物疗法的进展
- 皮肤病护理意识和可近性不断提高
- 产业陷阱与挑战
- 生物製剂和先进疗法成本高昂
- 副作用和依从性问题
- 成长动力
- 成长潜力分析
- 监管格局
- 差距分析
- 专利分析
- 管道分析
- 波特的分析
- PESTEL 分析
第四章:竞争格局
- 介绍
- 公司市占率分析
- 公司矩阵分析
- 主要市场参与者的竞争分析
- 竞争定位矩阵
- 策略仪表板
第五章:市场估计与预测:按药物类别,2021 年至 2034 年
- 主要趋势
- 皮质类固醇
- 钙调磷酸酶抑制剂
- 生物製剂
- 磷酸二酯酶-4 (PDE-4) 抑制剂
- 其他药物类别
第六章:市场估计与预测:依管理路线,2021 年至 2034 年
- 主要趋势
- 主题
- 口服
- 注射剂
第七章:市场估计与预测:依病患人口统计,2021 年至 2034 年
- 主要趋势
- 儿科
- 成年人
第八章:市场估计与预测:按配销通路,2021 年至 2034 年
- 主要趋势
- 医院药房
- 零售药局
- 电子商务
第九章:市场估计与预测:按地区,2021 年至 2034 年
- 主要趋势
- 北美洲
- 我们
- 加拿大
- 欧洲
- 德国
- 英国
- 法国
- 西班牙
- 义大利
- 荷兰
- 亚太地区
- 中国
- 日本
- 印度
- 澳洲
- 韩国
- 拉丁美洲
- 巴西
- 墨西哥
- 阿根廷
- 中东和非洲
- 南非
- 沙乌地阿拉伯
- 阿联酋
第十章:公司简介
- AbbVie
- Arcutis Biotherapeutics
- Eli Lilly and Company
- Galderma Laboratories
- Incyte Corporation
- Leo Pharma
- Maruho
- Novartis
- Otsuka Pharmaceutical
- Pfizer
- Regeneron Pharmaceuticals
- Sanofi
- Viatris
The Global Atopic Dermatitis Drugs Market was valued at USD 12.1 billion in 2024 and is projected to grow at a 9.9% CAGR from 2025 to 2034. Atopic dermatitis drugs help manage skin inflammation, itching, and dryness, targeting flare-ups and controlling the condition. These treatments include topical medications, systemic therapies, and biologics, chosen based on disease severity. The rising prevalence of atopic dermatitis, strong drug pipelines, and increased investment in research and development are fueling market growth. Pharmaceutical companies are launching new products, and positive clinical trial results are driving revenue expansion.

In 2023, the atopic dermatitis drugs market was worth USD 11.1 billion. The biologics segment led the market in 2024, holding a 41.3% revenue share, driven by higher efficacy and long-term symptom control. The demand for biologics continues to rise due to their superior treatment outcomes and growing product approvals.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $12.1 Billion |
| Forecast Value | $31 Billion |
| CAGR | 9.9% |
By route of administration, the market is divided into injectable, topical, and oral treatments. The injectable segment dominated in 2024 with a 45.1% revenue share. New biologic injections targeting inflammatory pathways, such as IL-4, IL-13, and JAK inhibitors, are expanding treatment options. Regulatory approvals have accelerated product launches, increasing accessibility and adoption rates in major markets.
The patient demographic segmentation includes pediatric and adult categories. The pediatric segment led with USD 8.7 billion in revenue in 2024, primarily due to the high incidence of atopic dermatitis in children. The demand for pediatric therapies continues to grow as regulatory bodies approve more treatments tailored for younger patients. Expedited approvals for biologic formulations specifically designed for pediatric use are further driving market expansion.
Distribution channels include hospital pharmacies, retail pharmacies, and e-commerce. Hospital pharmacies accounted for USD 5.8 billion in revenue in 2024. These pharmacies play a crucial role in managing patients who require specialized or systemic treatments, ensuring proper monitoring of therapy outcomes and potential side effects.
The U.S. market has witnessed significant growth, with revenue rising from USD 4.7 billion in 2023 to a projected USD 13.1 billion by 2034. Increased awareness efforts and advocacy initiatives are enhancing research funding and improving patient access to advanced treatment options. Regulatory agencies continue to fast-track the approval of biologics, particularly for severe cases and pediatric patients, further supporting market growth.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Base estimates and calculations
- 1.3.1 Base year calculation
- 1.3.2 Key trends for market estimation
- 1.4 Forecast model
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.5.2 Data mining sources
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Rising prevalence of atopic dermatitis
- 3.2.1.2 Advancements in biologic therapies
- 3.2.1.3 Growing awareness and access to dermatological care
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 High cost of biologic and advanced therapies
- 3.2.2.2 Side effects and compliance issues
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.5 Gap analysis
- 3.6 Patent analysis
- 3.7 Pipeline analysis
- 3.8 Porter’s analysis
- 3.9 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.3 Company matrix analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Strategy dashboard
Chapter 5 Market Estimates and Forecast, By Drug Class, 2021 – 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Corticosteroids
- 5.3 Calcineurin inhibitors
- 5.4 Biologics
- 5.5 Phosphodiesterase-4 (PDE-4) inhibitors
- 5.6 Other drug classes
Chapter 6 Market Estimates and Forecast, By Route of Administration, 2021 – 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Topical
- 6.3 Oral
- 6.4 Injectable
Chapter 7 Market Estimates and Forecast, By Patient Demographics, 2021 – 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Pediatric
- 7.3 Adults
Chapter 8 Market Estimates and Forecast, By Distribution Channel, 2021 – 2034 ($ Mn)
- 8.1 Key trends
- 8.2 Hospital pharmacies
- 8.3 Retail pharmacies
- 8.4 E-commerce
Chapter 9 Market Estimates and Forecast, By Region, 2021 – 2034 ($ Mn)
- 9.1 Key trends
- 9.2 North America
- 9.2.1 U.S.
- 9.2.2 Canada
- 9.3 Europe
- 9.3.1 Germany
- 9.3.2 UK
- 9.3.3 France
- 9.3.4 Spain
- 9.3.5 Italy
- 9.3.6 Netherlands
- 9.4 Asia Pacific
- 9.4.1 China
- 9.4.2 Japan
- 9.4.3 India
- 9.4.4 Australia
- 9.4.5 South Korea
- 9.5 Latin America
- 9.5.1 Brazil
- 9.5.2 Mexico
- 9.5.3 Argentina
- 9.6 Middle East and Africa
- 9.6.1 South Africa
- 9.6.2 Saudi Arabia
- 9.6.3 UAE
Chapter 10 Company Profiles
- 10.1 AbbVie
- 10.2 Arcutis Biotherapeutics
- 10.3 Eli Lilly and Company
- 10.4 Galderma Laboratories
- 10.5 Incyte Corporation
- 10.6 Leo Pharma
- 10.7 Maruho
- 10.8 Novartis
- 10.9 Otsuka Pharmaceutical
- 10.10 Pfizer
- 10.11 Regeneron Pharmaceuticals
- 10.12 Sanofi
- 10.13 Viatris










