 |
市场调查报告书
商品编码
1699286
合约研究组织 (CRO) 市场机会、成长动力、产业趋势分析及 2025-2034 年预测Contract Research Organization (CRO) Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025-2034 |
||||||
2023 年全球合约研究组织市场规模达到 598 亿美元,预计 2024 年至 2032 年期间的复合年增长率将达到 8.1%。随着製药和生物技术公司寻求具有成本效益的解决方案以加速药物开发并满足严格的监管要求,对外包研究服务的需求正在激增。随着全球临床试验数量的不断增长,CRO 在简化药物研发、降低营运成本和提高研究效率方面发挥着至关重要的作用。医学研究的投资持续增加,进一步加强了市场的成长轨迹。
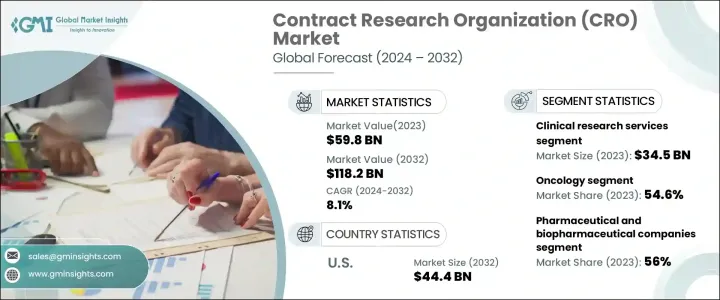
慢性病的日益普及推动了对创新治疗的需求,促使製药公司依赖专业研究公司。 CRO 提供临床试验、法规遵循和实验室服务的专业知识,使其成为製药和生物技术领域不可或缺的一部分。外包研究使公司能够专注于创新,同时利用 CRO 的基础设施和经验来应对复杂的监管环境。此外,精准医疗、基因疗法和生物製剂的进步正在扩大合约研究服务的范围,为产业参与者创造新的机会。全球向个人化医疗的转变进一步扩大了对专业研究的需求,CRO 促进了标靶治疗和尖端医疗解决方案的发展。
| 市场范围 | |
|---|---|
| 起始年份 | 2024 |
| 预测年份 | 2025-2034 |
| 起始值 | 598亿美元 |
| 预测值 | 1182亿美元 |
| 复合年增长率 | 8.1% |
市场按服务类型细分为早期开发、临床研究、实验室和监管咨询服务。临床研究服务引领产业发展,2023 年创造了 345 亿美元的收入。随着对创新治疗和诊断的需求不断增长,这一领域将继续蓬勃发展。临床研究分为四个阶段,每个阶段对于新疗法推向市场至关重要。慢性病病例的增加加剧了临床试验的需求,促使製药公司扩大研究力道。外包研究使公司能够优化资源、加快药物审批流程并提高临床试验的效率。
合约研究组织市场也依治疗领域分类,包括肿瘤学、心臟病学、传染病、神经病学、胃肠病学、肝病学、眼科学等。 2023 年,肿瘤学占据市场主导地位,占有 54.6% 的份额,预计到 2032 年将以 8.5% 的复合年增长率增长。癌症发生率的上升加大了开发先进疗法和医疗设备的研究力度,导致临床试验活动激增。对突破性治疗的追求正在推动对肿瘤学研究的投资,从而导致对合约研究服务的需求大幅增加。随着新疗法的出现,以肿瘤学为重点的试验正在迅速扩大,巩固了 CRO 在药物开发生态系统中的作用。
2023 年北美合约研究组织市场价值为 226 亿美元,预计到 2032 年将成长至 444 亿美元。该地区主要製药和生物技术公司的存在为其强大的市场份额做出了贡献。随着药物开发变得越来越复杂,对专业知识和最先进基础设施的需求也不断增长。 CRO 透过提供全面的研究解决方案、监管支援和数据驱动的见解,在推动医学研究方面发挥着重要作用,使其成为不断发展的製药领域的关键参与者。
目录
第一章:方法论与范围
第二章:执行摘要
第三章:行业洞察
- 产业生态系统分析
- 产业衝击力
- 成长动力
- 全球研发支出不断增加
- 新兴经济体临床试验数量不断增加
- 研发活动外包日益增多
- 不断进步的技术
- 产业陷阱与挑战
- 智慧财产权问题
- 成长动力
- 成长潜力分析
- 临床试验分析
- 2018 - 2023 年各地区临床试验数量
- 2018 - 2023 年临床试验数量(依开发阶段划分)
- 2018 - 2023 年临床试验量(依适应症划分)
- 监管格局
- 我们
- 欧洲
- 亚太地区
- 新加坡
- 马来西亚
- 印尼
- 泰国
- 韩国
- 菲律宾
- 临床试验-亚太优势
- 药物发现与开发过程
- 波特的分析
- PESTEL分析
第四章:竞争格局
- 介绍
- 公司矩阵分析
- 竞争市占率分析
- 策略仪表板
- 併购格局
- 2018 年至 2023 年 CRO 产业的併购交易
- 完成合约研究组织併购交易
- 私募股权在CRO併购中的影响
- 产业整合对整体市场的影响
- 分析师见解
第五章:市场估计与预测:按服务类型,2021 年至 2032 年
- 主要趋势
- 早期开发服务
- 发现阶段
- 化学、製造和控制(CMC)
- 临床前服务
- 药物动力学/药效动力学(PK/PD)
- 毒理学测试服务
- 其他临床前服务
- 临床研究服务
- 第一阶段
- 第二阶段
- 第三阶段
- 第四阶段
- 实验室服务
- 生物分析测试服务
- 分析测试服务
- 物理特性
- 原料检测
- 批次放行测试
- 稳定性测试
- 其他分析测试服务
- 监理咨询服务
第六章:市场估计与预测:按治疗领域,2021 年至 2032 年
- 主要趋势
- 肿瘤学
- 临床药理学
- 心臟病学
- 传染病
- 神经病学
- 胃肠病学和肝病学
- 眼科
- 其他治疗领域
第七章:市场估计与预测:依最终用途,2021 年至 2032 年
- 主要趋势
- 製药和生物製药公司
- 医疗器材公司
- 学术机构
第八章:市场估计与预测:按地区,2021 年至 2032 年
- 主要趋势
- 北美洲
- 我们
- 加拿大
- 欧洲
- 德国
- 英国
- 法国
- 西班牙
- 义大利
- 荷兰
- 亚太地区
- 中国
- 日本
- 印度
- 澳洲
- 韩国
- 拉丁美洲
- 巴西
- 墨西哥
- 中东和非洲
- 南非
- 沙乌地阿拉伯
- 阿联酋
第九章:公司简介
- Caidya
- Charles River Laboratories
- CMIC HOLDINGS
- EPS Holdings
- ICON plc
- IQVIA (Quintiles IMS)
- Iris Pharma
- Laboratory Corporation of America Holdings
- Lexitas Pharma Services
- Medpace Holdings
- Ora
- Parexel International (MA)
- Pharmaceutical Product Development (Thermo Fisher Scientific)
- Premier Research
- ProTrials Research
- Syneos Health
- TFS HealthScience
- The Emmes Company
- Trial Runners
- Veristat
- Worldwide Clinical Trials
- WuXi Clinical (WuXi AppTec)
The Global Contract Research Organization Market reached USD 59.8 billion in 2023 and is on track to expand at a CAGR of 8.1% from 2024 to 2032. The demand for outsourced research services is surging as pharmaceutical and biotechnology firms seek cost-effective solutions to accelerate drug development and meet stringent regulatory requirements. With a growing number of clinical trials worldwide, CROs are playing a crucial role in streamlining drug discovery, reducing operational costs, and enhancing research efficiency. Investments in medical research continue to rise, further strengthening the market's growth trajectory.

The increasing prevalence of chronic diseases is fueling the need for innovative treatments, driving pharmaceutical companies to rely on specialized research firms. CROs offer expertise in clinical trials, regulatory compliance, and laboratory services, making them indispensable to the pharmaceutical and biotech sectors. Outsourcing research allows companies to focus on innovation while leveraging the infrastructure and experience of CROs to navigate complex regulatory landscapes. Additionally, advancements in precision medicine, gene therapy, and biologics are expanding the scope of contract research services, creating new opportunities for industry players. The global shift toward personalized healthcare is further amplifying the demand for specialized research, with CROs facilitating the development of targeted therapies and cutting-edge medical solutions.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $59.8 Billion |
| Forecast Value | $118.2 Billion |
| CAGR | 8.1% |
The market is segmented by service type into early phase development, clinical research, laboratory, and regulatory consulting services. Clinical research services led the industry, generating USD 34.5 billion in revenue in 2023. This segment continues to thrive as the demand for innovative treatments and diagnostics rises. Clinical research is structured into four phases, each essential in bringing new therapies to market. The increase in chronic disease cases is intensifying the need for clinical trials, prompting pharmaceutical firms to expand their research efforts. Outsourcing research enables companies to optimize their resources, accelerate drug approval processes, and enhance efficiency in clinical trials.
The contract research organization market is also categorized by therapeutic area, including oncology, cardiology, infectious diseases, neurology, gastroenterology, hepatology, ophthalmology, and others. Oncology dominated the market in 2023 with a commanding 54.6% share and is expected to grow at a CAGR of 8.5% through 2032. The rising incidence of cancer has intensified research efforts to develop advanced therapies and medical devices, leading to an upsurge in clinical trial activity. The push for breakthrough treatments is driving investment in oncology research, leading to a significant increase in demand for contract research services. As new therapies emerge, oncology-focused trials are expanding rapidly, solidifying the role of CROs in the drug development ecosystem.
North America contract research organization market was valued at USD 22.6 billion in 2023, with projections indicating growth to USD 44.4 billion by 2032. The presence of major pharmaceutical and biotechnology firms in the region has contributed to its strong market share. As drug development becomes increasingly complex, the need for specialized expertise and state-of-the-art infrastructure continues to grow. CROs are instrumental in advancing medical research by providing comprehensive research solutions, regulatory support, and data-driven insights, positioning them as key players in the evolving pharmaceutical landscape.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Base estimates and calculations
- 1.3.1 Base year calculation
- 1.3.2 Key trends for market estimation
- 1.4 Forecast model
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.5.2 Data mining sources
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Increasing R&D expenditure worldwide
- 3.2.1.2 Rising number of clinical trials in emerging economies
- 3.2.1.3 Growing outsourcing of R&D activities
- 3.2.1.4 Rising technological advancements
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 Intellectual property right issues
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Clinical trial analysis
- 3.4.1 Clinical trial volume, by region, 2018 - 2023
- 3.4.2 Clinical trial volume, by phase of development, 2018 - 2023
- 3.4.3 Clinical trial volume, by indication, 2018 - 2023
- 3.5 Regulatory landscape
- 3.5.1 U.S.
- 3.5.2 Europe
- 3.5.3 Asia Pacific
- 3.5.3.1 Singapore
- 3.5.3.2 Malaysia
- 3.5.3.3 Indonesia
- 3.5.3.4 Thailand
- 3.5.3.5 South Korea
- 3.5.3.6 Philippines
- 3.6 Clinical trials- Asia Pacific advantage
- 3.7 Drug discovery and development process
- 3.8 Porter's analysis
- 3.9 PESTEL analysis
Chapter 4 Competitive Landscape, 2023
- 4.1 Introduction
- 4.2 Company matrix analysis
- 4.3 Competitive market share analysis
- 4.4 Strategy dashboard
- 4.5 Merger and acquisition landscape
- 4.5.1 Merger and acquisition deals in the CRO industry, 2018 – 2023
- 4.5.2 Completed contract research organization merger and acquisition deals, 2018 - 2023
- 4.5.3 Influence of private equity in CRO mergers and acquisitions
- 4.5.4 Impact of industry consolidation on the overall market
- 4.5.5 Analyst insights
Chapter 5 Market Estimates and Forecast, By Service Type, 2021 – 2032 ($ Mn)
- 5.1 Key trends
- 5.2 Early phase development services
- 5.2.1 Discovery phase
- 5.2.2 Chemistry, manufacturing and control (CMC)
- 5.2.3 Preclinical services
- 5.2.3.1 Pharmacokinetic/pharmacodynamic (PK/PD)
- 5.2.3.2 Toxicology testing services
- 5.2.3.3 Other preclinical services
- 5.3 Clinical research services
- 5.3.1 Phase I
- 5.3.2 Phase II
- 5.3.3 Phase III
- 5.3.4 Phase IV
- 5.4 Laboratory services
- 5.4.1 Bioanalytical testing services
- 5.4.2 Analytical testing services
- 5.4.2.1 Physical characterization
- 5.4.2.2 Raw material testing
- 5.4.2.3 Batch release testing
- 5.4.2.4 Stability testing
- 5.4.2.5 Other analytical testing services
- 5.5 Regulatory consulting services
Chapter 6 Market Estimates and Forecast, By Therapeutic Area, 2021 – 2032 ($ Mn)
- 6.1 Key trends
- 6.2 Oncology
- 6.3 Clinical pharmacology
- 6.4 Cardiology
- 6.5 Infectious disease
- 6.6 Neurology
- 6.7 Gastroenterology and hepatology
- 6.8 Ophthalmology
- 6.9 Other therapeutic areas
Chapter 7 Market Estimates and Forecast, By End Use, 2021 – 2032 ($ Mn)
- 7.1 Key trends
- 7.2 Pharmaceutical and biopharmaceutical companies
- 7.3 Medical device companies
- 7.4 Academic institutes
Chapter 8 Market Estimates and Forecast, By Region, 2021 – 2032 ($ Mn)
- 8.1 Key trends
- 8.2 North America
- 8.2.1 U.S.
- 8.2.2 Canada
- 8.3 Europe
- 8.3.1 Germany
- 8.3.2 UK
- 8.3.3 France
- 8.3.4 Spain
- 8.3.5 Italy
- 8.3.6 Netherlands
- 8.4 Asia Pacific
- 8.4.1 China
- 8.4.2 Japan
- 8.4.3 India
- 8.4.4 Australia
- 8.4.5 South Korea
- 8.5 Latin America
- 8.5.1 Brazil
- 8.5.2 Mexico
- 8.6 Middle East and Africa
- 8.6.1 South Africa
- 8.6.2 Saudi Arabia
- 8.6.3 UAE
Chapter 9 Company Profiles
- 9.1 Caidya
- 9.2 Charles River Laboratories
- 9.3 CMIC HOLDINGS
- 9.4 EPS Holdings
- 9.5 ICON plc
- 9.6 IQVIA (Quintiles IMS)
- 9.7 Iris Pharma
- 9.8 Laboratory Corporation of America Holdings
- 9.9 Lexitas Pharma Services
- 9.10 Medpace Holdings
- 9.11 Ora
- 9.12 Parexel International (MA)
- 9.13 Pharmaceutical Product Development (Thermo Fisher Scientific)
- 9.14 Premier Research
- 9.15 ProTrials Research
- 9.16 Syneos Health
- 9.17 TFS HealthScience
- 9.18 The Emmes Company
- 9.19 Trial Runners
- 9.20 Veristat
- 9.21 Worldwide Clinical Trials
- 9.22 WuXi Clinical (WuXi AppTec)










