 |
市场调查报告书
商品编码
1708182
肿瘤伴随诊断市场机会、成长动力、产业趋势分析及 2025 - 2034 年预测Oncology Companion Diagnostic Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
2024 年全球肿瘤伴随诊断市场规模达 46 亿美元,预计 2025 年至 2034 年的复合年增长率为 10.8%。肿瘤伴随诊断是提供关键见解的重要工具,可确保在癌症治疗中安全有效地使用相应药物或生物製品。这些诊断有助于识别患者肿瘤中的特定基因标记、突变和生物标记,使医疗保健提供者能够确定患者是否可能对标靶治疗产生反应。精准医疗的日益普及已将重点从传统治疗方法转移到根据患者独特的肿瘤特征和基因特征定制治疗的个人化方法。这一趋势是推动肿瘤伴随诊断需求的关键驱动因素。
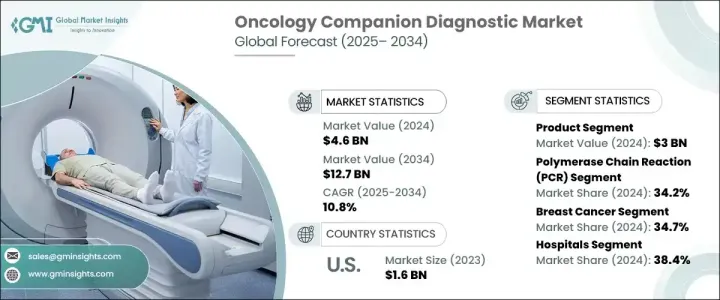
市场根据产品、技术、疾病类型和最终用途进行细分。根据提供内容,市场分为产品和服务。该产品部门包括仪器、消耗品和软体,2024 年创造了 30 亿美元的收入,预计在预测期内的复合年增长率为 10.6%。聚合酶炼式反应 (PCR) 系统和次世代定序仪 (NGS) 等仪器在检测基因突变方面具有很高的灵敏度和准确性。消耗品(包括试剂、检测试剂盒、引子和探针)可确保结果的准确性。软体解决方案在分析和解释复杂的基因组和临床资料方面发挥关键作用,人工智慧 (AI) 和机器学习 (ML) 等先进演算法可以提高预测准确性并实现简化的资料解释。
| 市场范围 | |
|---|---|
| 起始年份 | 2024 |
| 预测年份 | 2025-2034 |
| 起始值 | 46亿美元 |
| 预测值 | 127亿美元 |
| 复合年增长率 | 10.8% |
根据技术,市场还分为 PCR、NGS、免疫组织化学 (IHC)、原位杂交 (ISH)/萤光原位杂交 (FISH) 和其他技术。 PCR 在 2024 年占据了 34.2% 的市场份额,预计到 2034 年将达到 45 亿美元。 PCR 系统在检测微量 DNA 或 RNA 方面具有高灵敏度和效率,可在数小时内快速提供结果,这对于及时的临床决策至关重要。 PCR 技术的进步,例如数位 PCR (dPCR) 和即时 PCR (qPCR),使得可以在一次测试中同时检测多种癌症生物标记物,从而降低成本并节省时间。
依疾病类型,市场分为乳癌、非小细胞肺癌、大肠癌、白血病、黑色素瘤、摄护腺癌等。乳癌领域在 2024 年占据市场主导地位,占有 34.7% 的份额,这主要是由于疾病的盛行率不断上升。最终用途细分包括医院、诊断实验室、学术和研究机构以及其他最终用户。 2024 年,医院占据了 38.4% 的市场份额,这归功于其管理大量住院和门诊癌症患者的能力。伴随诊断使医院能够管理个人化治疗,减少副作用并提高存活率。
由于癌症发病率不断上升以及美国食品药物管理局(FDA)对伴随诊断的严格标准,美国仍然是一个关键市场。这些法规鼓励开发更安全、更有效的诊断试剂盒和仪器,促进该地区肿瘤伴随诊断市场的整体成长。
目录
第一章:方法论与范围
第二章:执行摘要
第三章:行业洞察
- 产业生态系统分析
- 产业衝击力
- 成长动力
- 癌症发生率上升
- 基因组技术的进步
- 标靶治疗需求不断增长
- 增加研发投入
- 产业陷阱与挑战
- 开发成本高
- 严格的监管要求
- 成长动力
- 成长潜力分析
- 监管格局
- 技术格局
- 未来市场趋势
- 波特的分析
- PESTEL分析
第四章:竞争格局
- 介绍
- 公司市占率分析
- 公司矩阵分析
- 主要市场参与者的竞争分析
- 竞争定位矩阵
- 策略仪表板
第五章:市场估计与预测:依供应量,2021 年至 2034 年
- 主要趋势
- 产品
- 乐器
- 耗材
- 软体
- 服务
第六章:市场估计与预测:按技术,2021 年至 2034 年
- 主要趋势
- 聚合酶炼式反应(PCR)
- 下一代定序(NGS)
- 免疫组织化学(IHC)
- 原位杂交(ISH)/萤光原位杂交(FISH)
- 其他技术
第七章:市场估计与预测:依疾病类型,2021 年至 2034 年
- 主要趋势
- 乳癌
- 非小细胞肺癌
- 大肠直肠癌
- 白血病
- 黑色素瘤
- 摄护腺癌
- 其他疾病类型
第八章:市场估计与预测:依最终用途,2021 年至 2034 年
- 主要趋势
- 医院
- 诊断实验室
- 学术和研究机构
- 其他最终用途
第九章:市场估计与预测:按地区,2021 年至 2034 年
- 主要趋势
- 北美洲
- 我们
- 加拿大
- 欧洲
- 德国
- 英国
- 法国
- 西班牙
- 义大利
- 荷兰
- 亚太地区
- 中国
- 日本
- 印度
- 澳洲
- 韩国
- 拉丁美洲
- 巴西
- 墨西哥
- 阿根廷
- 中东和非洲
- 南非
- 沙乌地阿拉伯
- 阿联酋
第十章:公司简介
- Abbott Laboratories
- Agilent Technologies
- BioMérieux
- Biocartis
- Bio-Rad Laboratories
- EntroGen
- F. Hoffmann-La Roche
- Foundation Medicine
- Guardant Health
- Illumina
- Leica Biosystems
- Myriad Genetics
- QIAGEN
- Sysmex Corporation
- Thermo Fisher Scientific
The Global Oncology Companion Diagnostic Market reached USD 4.6 billion in 2024 and is expected to grow at a CAGR of 10.8% from 2025 to 2034. Oncology companion diagnostics are essential tools that provide critical insights into the safe and effective use of corresponding drugs or biological products in cancer treatment. These diagnostics help identify specific genetic markers, mutations, and biomarkers in a patient's tumor, enabling healthcare providers to determine whether a patient is likely to respond to a targeted therapy. The increasing adoption of precision medicine has shifted the focus from traditional treatment methods to personalized approaches that tailor therapies based on a patient's unique tumor characteristics and genetic profile. This trend is a key driver fueling the demand for oncology companion diagnostics.

The market is segmented by offering, technology, disease type, and end-use. By offering, the market is divided into products and services. The product segment, which includes instruments, consumables, and software, generated USD 3 billion in revenue in 2024 and is projected to grow at a CAGR of 10.6% during the forecast period. Instruments such as polymerase chain reaction (PCR) systems and next-generation sequencers (NGS) offer high sensitivity and accuracy in detecting genetic mutations. Consumables, including reagents, assay kits, primers, and probes, ensure accurate results. Software solutions play a pivotal role in analyzing and interpreting complex genomic and clinical data, with advanced algorithms such as artificial intelligence (AI) and machine learning (ML) improving predictive accuracy and enabling streamlined data interpretation.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $4.6 Billion |
| Forecast Value | $12.7 Billion |
| CAGR | 10.8% |
The market is also categorized by technology into PCR, NGS, immunohistochemistry (IHC), in situ hybridization (ISH)/fluorescence in situ hybridization (FISH), and other technologies. PCR held a 34.2% market share in 2024 and is expected to reach USD 4.5 billion by 2034. PCR systems provide high sensitivity and efficiency in detecting minimal amounts of DNA or RNA, delivering quick results within hours, which is essential for timely clinical decision-making. Advancements in PCR technologies, such as digital PCR (dPCR) and real-time PCR (qPCR), allow for the simultaneous detection of multiple cancer biomarkers in a single test, reducing costs and saving time.
By disease type, the market is divided into breast cancer, non-small cell lung cancer, colorectal cancer, leukemia, melanoma, prostate cancer, and others. The breast cancer segment dominated the market with a 34.7% share in 2024, primarily due to the rising prevalence of the disease. End use segmentation includes hospitals, diagnostic laboratories, academic and research institutions, and other end users. Hospitals accounted for 38.4% of the market share in 2024, attributed to their capacity to manage high volumes of cancer patients, both inpatient and outpatient. Companion diagnostics empower hospitals to manage personalized treatments, reducing side effects and improving survival rates.
The United States remains a key market due to the increasing incidence of cancer and the stringent standards established by the U.S. Food and Drug Administration (FDA) for companion diagnostics. These regulations have encouraged the development of safer, more effective diagnostic kits and instruments, enhancing the overall growth of the oncology companion diagnostic market in the region.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Base estimates and calculations
- 1.3.1 Base year calculation
- 1.3.2 Key trends for market estimation
- 1.4 Forecast model
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.5.2 Data mining sources
Chapter 2 Executive Summary
- 2.1 Industry 360° synopsis
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Rising prevalence of cancer
- 3.2.1.2 Advancements in genomic technologies
- 3.2.1.3 Rising demand for targeted therapies
- 3.2.1.4 Increased investment in research and development
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 High development costs
- 3.2.2.2 Stringent regulatory requirements
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.5 Technological landscape
- 3.6 Future market trends
- 3.7 Porter's analysis
- 3.8 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.3 Company matrix analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Strategy dashboard
Chapter 5 Market Estimates and Forecast, By Offering, 2021 – 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Product
- 5.2.1 Instrument
- 5.2.2 Consumables
- 5.2.3 Software
- 5.3 Services
Chapter 6 Market Estimates and Forecast, By Technology, 2021 – 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Polymerase chain reaction (PCR)
- 6.3 Next-generation sequencing (NGS)
- 6.4 Immunohistochemistry (IHC)
- 6.5 In situ hybridization (ISH)/fluorescence in situ hybridization (FISH)
- 6.6 Other technologies
Chapter 7 Market Estimates and Forecast, By Disease Type, 2021 – 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Breast cancer
- 7.3 Non-small cell lung cancer
- 7.4 Colorectal cancer
- 7.5 Leukemia
- 7.6 Melanoma
- 7.7 Prostate cancer
- 7.8 Other disease types
Chapter 8 Market Estimates and Forecast, By End Use, 2021 – 2034 ($ Mn)
- 8.1 Key trends
- 8.2 Hospitals
- 8.3 Diagnostic laboratories
- 8.4 Academic and research institutions
- 8.5 Other end use
Chapter 9 Market Estimates and Forecast, By Region, 2021 – 2034 ($ Mn)
- 9.1 Key trends
- 9.2 North America
- 9.2.1 U.S.
- 9.2.2 Canada
- 9.3 Europe
- 9.3.1 Germany
- 9.3.2 UK
- 9.3.3 France
- 9.3.4 Spain
- 9.3.5 Italy
- 9.3.6 Netherlands
- 9.4 Asia Pacific
- 9.4.1 China
- 9.4.2 Japan
- 9.4.3 India
- 9.4.4 Australia
- 9.4.5 South Korea
- 9.5 Latin America
- 9.5.1 Brazil
- 9.5.2 Mexico
- 9.5.3 Argentina
- 9.6 Middle East and Africa
- 9.6.1 South Africa
- 9.6.2 Saudi Arabia
- 9.6.3 UAE
Chapter 10 Company Profiles
- 10.1 Abbott Laboratories
- 10.2 Agilent Technologies
- 10.3 BioMérieux
- 10.4 Biocartis
- 10.5 Bio-Rad Laboratories
- 10.6 EntroGen
- 10.7 F. Hoffmann-La Roche
- 10.8 Foundation Medicine
- 10.9 Guardant Health
- 10.10 Illumina
- 10.11 Leica Biosystems
- 10.12 Myriad Genetics
- 10.13 QIAGEN
- 10.14 Sysmex Corporation
- 10.15 Thermo Fisher Scientific








