 |
市场调查报告书
商品编码
1708209
临床试验包装市场机会、成长动力、产业趋势分析及 2025 - 2034 年预测Clinical Trial Packaging Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
2024 年全球临床试验包装市场规模达 37 亿美元,预计 2025 年至 2034 年的复合年增长率为 10.2%,这主要得益于对生物製剂、复杂药物配方的需求不断增长以及全球范围内进行的临床试验数量不断增加。随着製药业的发展,出现了明显的朝向更小、更客製化批次发展的趋势,对灵活、可扩展的包装解决方案的需求强烈。人们对个人化医疗和标靶治疗(包括基因疗法和 mRNA 疫苗)的日益关注,加速了对确保产品在运输和储存过程中稳定性和安全性的专用包装的需求。
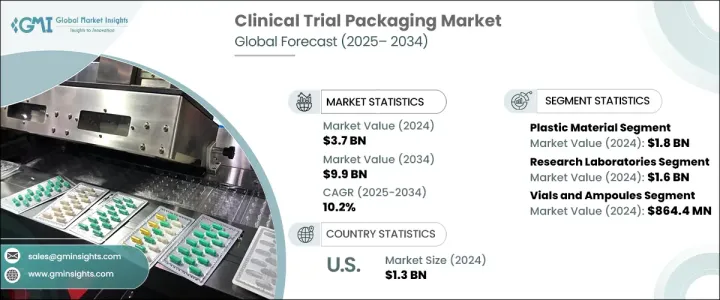
此外,分散临床试验 (DCT) 的出现增加了包装要求的复杂性,製造商优先考虑多功能、易于管理的解决方案,以满足不同的试验形式。严格的监管标准进一步强调了包装的必要性,即保持温度敏感生物製剂的完整性,同时确保符合国际安全准则。包装技术的进步,包括使用配备即时监控功能的智慧包装系统,正在提高临床试验供应链的效率和可靠性。
| 市场范围 | |
|---|---|
| 起始年份 | 2024 |
| 预测年份 | 2025-2034 |
| 起始值 | 37亿美元 |
| 预测值 | 99亿美元 |
| 复合年增长率 | 10.2% |
市场按材料类型细分,包括塑胶、玻璃、金属和纸质包装。其中,塑胶领域由于其重量轻、成本低廉的特性,非常适合大规模运输和储存,因此在 2024 年创造了 18 亿美元的市场价值。塑胶包装适应性强,製造商可以轻鬆客製化临床试验套件和小批量格式。这种多功能性对于容纳预充式註射器、小袋和其他需要灵活性和易于生产的药品特别有益。随着製药业对包装的精确性和多样性要求越来越高,塑胶仍然是支持广泛临床试验包装要求的首选。
研究实验室成为临床试验包装市场中最大的终端使用者群体,2024 年的市场规模达到 16 亿美元。这些实验室在临床试验生态系统中发挥关键作用,尤其是在药物开发的早期阶段,需要对少量各种药物配方进行专门的包装。人们对生物製剂和个人化治疗(包括基因治疗和标靶治疗)的日益重视进一步推动了对无菌、温度敏感包装解决方案的需求。玻璃小瓶和低温容器对于维持敏感製剂的稳定性、确保临床试验期间药物的安全性和有效性尤其重要。
2024 年,北美占据临床试验包装市场 41.5% 的主导份额,这得益于个人化医疗、生物製剂和罕见疾病治疗临床试验数量的不断增加。政府对分散临床试验和冷链基础设施的投资正在加速整个地区对专业包装解决方案的需求。慢性病盛行率的上升,加上生物製剂和细胞疗法的不断进步,促使製药公司大力投资创新包装解决方案,以满足不断变化的临床试验要求。这些发展预计将推动临床试验包装行业的进一步成长和创新,特别是在北美,那里强大的医疗保健基础设施和监管支持为市场扩张创造了有利的环境。
目录
第一章:方法论与范围
第二章:执行摘要
第三章:行业洞察
- 产业生态系统分析
- 产业衝击力
- 成长动力
- 製药公司加大研发投入
- 严格的药品安全包装规定
- 生物製剂和复杂药物製剂的成长
- 临床试验数量不断增加
- 慢性病盛行率上升
- 产业陷阱与挑战
- 专业包装成本高
- 冷链管理中的物流限制
- 成长动力
- 成长潜力分析
- 监管格局
- 技术格局
- 未来市场趋势
- 差距分析
- 波特的分析
- PESTEL分析
第四章:竞争格局
- 介绍
- 公司市占率分析
- 主要市场参与者的竞争分析
- 竞争定位矩阵
- 策略仪表板
第五章:市场估计与预测:按材料类型,2021 - 2034 年
- 主要趋势
- 塑胶
- 玻璃
- 金属
- 纸和纸板
第六章:市场估计与预测:依产品类型,2021 - 2034 年
- 主要趋势
- 注射器
- 小瓶和安瓿瓶
- 水泡
- 管
- 瓶子
- 包包和小袋
- 套件和包装
- 其他的
第七章:市场估计与预测:依最终用途,2021 - 2034 年
- 主要趋势
- 研究实验室
- 临床研究组织
- 药品生产设施
第八章:市场估计与预测:按地区,2021 - 2034 年
- 主要趋势
- 北美洲
- 我们
- 加拿大
- 欧洲
- 德国
- 英国
- 法国
- 西班牙
- 义大利
- 荷兰
- 亚太地区
- 中国
- 印度
- 日本
- 澳洲
- 韩国
- 拉丁美洲
- 巴西
- 墨西哥
- 阿根廷
- 中东和非洲
- 沙乌地阿拉伯
- 南非
- 阿联酋
第九章:公司简介
- Adare Pharma Solutions
- Adelphi Healthcare Packaging
- Almac Group
- Amcor
- AptarGroup
- Berry Global
- Brentwood Industries
- Caprihans India
- CCL Industries
- DWK Life Sciences
- Gerresheimer
- Korber
- Parexel International
- Schott
- SGD Pharma
- Sharp Services
- TA Instruments
- WestRock
- Yourway
The Global Clinical Trial Packaging Market generated USD 3.7 billion in 2024 and is anticipated to grow at a CAGR of 10.2% from 2025 to 2034, driven by increasing demand for biologics, complex drug formulations, and a growing number of clinical trials conducted worldwide. As the pharmaceutical industry evolves, there is a clear trend toward smaller, more customized batches, creating a strong need for flexible and scalable packaging solutions. The rising focus on personalized medicine and targeted therapies, including gene therapies and mRNA vaccines, is accelerating the demand for specialized packaging that ensures product stability and safety during transportation and storage.

Additionally, the emergence of decentralized clinical trials (DCTs) is adding complexity to packaging requirements, with manufacturers prioritizing versatile, easy-to-manage solutions that cater to diverse trial formats. Stringent regulatory standards further emphasize the need for packaging that maintains the integrity of temperature-sensitive biologics while ensuring compliance with international safety guidelines. Advancements in packaging technologies, including the use of smart packaging systems equipped with real-time monitoring features, are enhancing the efficiency and reliability of clinical trial supply chains.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $3.7 Billion |
| Forecast Value | $9.9 Billion |
| CAGR | 10.2% |
The market is segmented by material type, including plastic, glass, metal, and paper-based packaging. Among these, the plastic segment generated USD 1.8 billion in 2024, owing to its lightweight, cost-effective nature, making it ideal for large-scale shipping and storage. Plastic packaging is highly adaptable, allowing manufacturers to customize clinical trial kits and small batch formats easily. This versatility is especially beneficial for accommodating prefilled syringes, pouches, and other pharmaceutical products that require flexibility and ease of production. As the pharmaceutical landscape increasingly demands precision and variability in packaging, plastic remains the preferred choice for supporting a wide range of clinical trial packaging requirements.
Research laboratories emerged as the largest end-user segment in the clinical trial packaging market, generating USD 1.6 billion in 2024. These laboratories play a pivotal role in the clinical trial ecosystem, particularly during the early stages of drug development, where specialized packaging is required for small quantities of various drug formulations. The growing emphasis on biologics and personalized therapies, including gene therapies and targeted treatments, has further fueled the demand for sterile, temperature-sensitive packaging solutions. Glass vials and cryogenic containers are particularly essential for preserving the stability of sensitive formulations, ensuring the safety and efficacy of drugs during clinical trials.
North America held a dominant 41.5% share of the clinical trial packaging market in 2024, driven by the increasing number of clinical trials focused on personalized medicine, biologics, and treatments for rare diseases. Government investments in decentralized clinical trials and cold chain infrastructure are accelerating the demand for specialized packaging solutions across the region. The rising prevalence of chronic diseases, coupled with ongoing advancements in biologics and cell-based therapies, is prompting pharmaceutical companies to invest heavily in innovative packaging solutions to meet evolving clinical trial requirements. These developments are expected to fuel further growth and innovation in the clinical trial packaging industry, particularly in North America, where a robust healthcare infrastructure and regulatory support create a conducive environment for market expansion.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Base estimates and calculations
- 1.3.1 Base year calculation
- 1.3.2 Key trends for market estimation
- 1.4 Forecast model
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.5.2 Data mining sources
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Increasing R&D investment by pharmaceutical companies
- 3.2.1.2 Stringent packaging regulations for drug safety
- 3.2.1.3 Growth in biologics and complex drug formulations
- 3.2.1.4 Growing number of clinical trials
- 3.2.1.5 Rising prevalence of chronic diseases
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 High cost of specialized packaging
- 3.2.2.2 Logistical constraints in cold chain management
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.5 Technology landscape
- 3.6 Future market trends
- 3.7 Gap analysis
- 3.8 Porter's analysis
- 3.9 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.3 Competitive analysis of major market players
- 4.4 Competitive positioning matrix
- 4.5 Strategy dashboard
Chapter 5 Market Estimates and Forecast, By Material Type, 2021 - 2034 (USD Million & Kilo Tons)
- 5.1 Key trends
- 5.2 Plastic
- 5.3 Glass
- 5.4 Metal
- 5.5 Paper & paperboard
Chapter 6 Market Estimates and Forecast, By Product Type, 2021 - 2034 (USD Million & Kilo Tons)
- 6.1 Key trends
- 6.2 Syringes
- 6.3 Vials & ampoules
- 6.4 Blisters
- 6.5 Tubes
- 6.6 Bottles
- 6.7 Bags & pouches
- 6.8 Kits and packs
- 6.9 Others
Chapter 7 Market Estimates and Forecast, By End Use, 2021 - 2034 (USD Million & Kilo Tons)
- 7.1 Key trends
- 7.2 Research laboratories
- 7.3 Clinical research organizations
- 7.4 Drug manufacturing facilities
Chapter 8 Market Estimates and Forecast, By Region, 2021 - 2034 (USD Million & Kilo Tons)
- 8.1 Key trends
- 8.2 North America
- 8.2.1 U.S.
- 8.2.2 Canada
- 8.3 Europe
- 8.3.1 Germany
- 8.3.2 UK
- 8.3.3 France
- 8.3.4 Spain
- 8.3.5 Italy
- 8.3.6 Netherlands
- 8.4 Asia Pacific
- 8.4.1 China
- 8.4.2 India
- 8.4.3 Japan
- 8.4.4 Australia
- 8.4.5 South Korea
- 8.5 Latin America
- 8.5.1 Brazil
- 8.5.2 Mexico
- 8.5.3 Argentina
- 8.6 Middle East and Africa
- 8.6.1 Saudi Arabia
- 8.6.2 South Africa
- 8.6.3 UAE
Chapter 9 Company Profiles
- 9.1 Adare Pharma Solutions
- 9.2 Adelphi Healthcare Packaging
- 9.3 Almac Group
- 9.4 Amcor
- 9.5 AptarGroup
- 9.6 Berry Global
- 9.7 Brentwood Industries
- 9.8 Caprihans India
- 9.9 CCL Industries
- 9.10 DWK Life Sciences
- 9.11 Gerresheimer
- 9.12 Korber
- 9.13 Parexel International
- 9.14 Schott
- 9.15 SGD Pharma
- 9.16 Sharp Services
- 9.17 TA Instruments
- 9.18 WestRock
- 9.19 Yourway







