 |
市场调查报告书
商品编码
1721607
体外诊断市场机会、成长动力、产业趋势分析及 2025 - 2034 年预测In-vitro Diagnostics Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
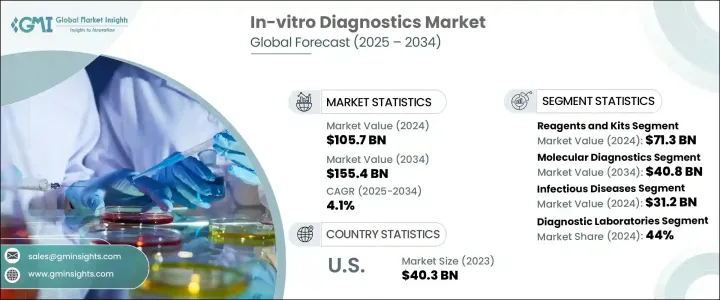
2024 年全球体外诊断市场价值为 1,057 亿美元,预计到 2034 年将以 4.1% 的复合年增长率成长,达到 1,554 亿美元。体外诊断 (IVD) 包括用于分析人体外部血液、尿液和组织等生物样本的各种医疗设备、试剂和系统。这些工具在检测、诊断和管理疾病方面发挥着至关重要的作用,可以做出更快、更准确的临床决策。随着世界各地的医疗保健系统不断向预防保健和精准医疗转变,对先进诊断工具的需求正在稳步上升。
IVD 解决方案不仅在医院和临床实验室中得到越来越广泛的应用,而且在家庭护理和护理点环境中也得到越来越广泛的应用。慢性病和传染病负担的不断加重、人们对早期疾病检测认识的不断提高以及新型诊断技术的出现共同推动了这个市场的扩张。此外,人工智慧和数位健康平台在诊断中的应用正在改变结果的生成、解释和传递方式,为个人化和即时护理创造了新的途径。支持性的监管政策、有利的报销方案以及已开发和新兴经济体对医疗数位化的强力推动进一步加速了该产业的成长轨迹。
| 市场范围 | |
|---|---|
| 起始年份 | 2024 |
| 预测年份 | 2025-2034 |
| 起始值 | 1057亿美元 |
| 预测值 | 1554亿美元 |
| 复合年增长率 | 4.1% |
试剂和试剂盒领域在 2024 年创造了 713 亿美元的产值,预计在 2025 年至 2034 年期间的复合年增长率将达到 4.4%。这一增长主要得益于对精准和早期诊断日益增长的需求,以及对客製化治疗方案日益增长的需求。试剂和试剂盒涵盖广泛的产品,包括生化试剂、抗体、检测试剂盒、探针和引物,所有这些都是运行各种诊断测试所必需的。随着分子诊断技术的进步,这些产品现在能够提供比传统检测方法更准确、更快速的结果,使其成为现代诊断实验室中不可或缺的一部分。
分子诊断领域在 2024 年占了 25.5% 的市场份额,预计将大幅成长,到 2034 年达到 408 亿美元。该领域受益于不断提高测试准确性、週转时间和易用性的创新。分子诊断工具由于能够在分子层面上识别病原体和基因突变,现已成为检测传染病、遗传疾病和癌症的首选。与通常需要几天才能返回结果的传统测试不同,分子诊断可以在几小时甚至几分钟内产生可操作的见解,从而能够更快地做出治疗决策并改善患者的治疗效果。
2024 年,北美体外诊断市场规模达到 451 亿美元,凭藉强大的医疗基础设施、较高的慢性病负担以及早期采用先进技术,保持主导地位。儘管监管环境严格,该地区仍继续支持尖端诊断解决方案的批准和商业化。
全球体外诊断市场的主要参与者包括雅培实验室、ACON 实验室、安捷伦科技、碧迪公司、Bio-Rad 实验室、Dragerwerk、罗氏公司、丹纳赫集团、美敦力、Meridian Bioscience、Nova Biomedical、珀金埃尔默、西门子医疗和 Sysmex 公司。这些公司专注于研发、策略合作伙伴关係和全球扩张,以满足对先进诊断技术日益增长的需求。
目录
第一章:方法论与范围
第二章:执行摘要
第三章:行业洞察
- 产业生态系统分析
- 产业衝击力
- 成长动力
- 全球传染病和慢性病发病率不断上升
- 个人化医疗的采用率不断提高,需求不断成长
- 发展中国家越来越多地采用即时检测
- 配备先进诊断仪器的病理实验室和服务数量激增
- 产业陷阱与挑战
- 诊断服务费用高昂
- 不利的报销情况
- 成长动力
- 成长潜力分析
- 监管格局
- 技术格局
- 未来市场趋势
- 差距分析
- 波特的分析
- PESTEL分析
第四章:竞争格局
- 介绍
- 公司市占率分析
- 公司矩阵分析
- 主要市场参与者的竞争分析
- 竞争定位矩阵
- 策略仪表板
第五章:市场估计与预测:按产品,2021 - 2034 年
- 主要趋势
- 试剂和试剂盒
- 仪器
第六章:市场估计与预测:按测试类型,2021 - 2034 年
- 主要趋势
- 临床化学
- 免疫测定/免疫化学
- 分子诊断
- 血液学
- 尿液分析
- 其他测试类型
第七章:市场估计与预测:按应用,2021 - 2034 年
- 主要趋势
- 肿瘤学
- 传染病
- 糖尿病
- 心臟病学
- 肾臟病学
- 自体免疫疾病
- 药物检测/药物基因组学
- 其他应用
第八章:市场估计与预测:依最终用途,2021 - 2034 年
- 主要趋势
- 医院
- 诊断实验室
- 学术和研究机构
- 其他最终用途
第九章:市场估计与预测:按地区,2021 - 2034 年
- 主要趋势
- 北美洲
- 我们
- 加拿大
- 欧洲
- 德国
- 英国
- 法国
- 西班牙
- 义大利
- 亚太地区
- 中国
- 印度
- 日本
- 澳洲
- 韩国
- 拉丁美洲
- 巴西
- 墨西哥
- 阿根廷
- 中东和非洲
- 沙乌地阿拉伯
- 南非
- 阿联酋
第十章:公司简介
- Abbott Laboratories
- ACON Laboratories
- Agilent Technologies
- Becton, Dickinson and Company
- Biomerieux
- Bio-Rad Laboratories
- Dragerwerk
- F. Hoffmann-La Roche
- Danaher Corporation
- Medtronic
- Meridian Bioscience
- Nova Biomedical
- PerkinElmer
- Siemens Healthineers
- Sysmex Corporation
The Global In-Vitro Diagnostics Market was valued at USD 105.7 billion in 2024 and is estimated to grow at a CAGR of 4.1% to reach USD 155.4 billion by 2034. In-vitro diagnostics (IVD) includes a wide range of medical devices, reagents, and systems used to analyze biological samples such as blood, urine, and tissues outside the human body. These tools play a vital role in detecting, diagnosing, and managing diseases, enabling faster and more accurate clinical decisions. As healthcare systems around the world continue to shift toward preventive care and precision medicine, the demand for advanced diagnostic tools is rising steadily.

IVD solutions are increasingly being adopted not only in hospitals and clinical laboratories but also in home care and point-of-care settings. The growing burden of chronic and infectious diseases, rising awareness about early disease detection, and the emergence of novel diagnostic technologies are collectively driving the expansion of this market. Moreover, the adoption of artificial intelligence and digital health platforms in diagnostics is transforming the way results are generated, interpreted, and delivered, creating new avenues for personalized and real-time care. Supportive regulatory policies, favorable reimbursement scenarios, and a strong push for healthcare digitization across developed and emerging economies further accelerate the industry's growth trajectory.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $105.7 Billion |
| Forecast Value | $155.4 Billion |
| CAGR | 4.1% |
The reagents and kits segment generated USD 71.3 billion in 2024 and is expected to grow at a CAGR of 4.4% from 2025 to 2034. This growth is largely driven by the increasing need for precise and early-stage diagnosis, as well as the rising demand for customized treatment plans. Reagents and kits cover a broad spectrum of products, including biochemical reagents, antibodies, assay kits, probes, and primers, all of which are essential for running a variety of diagnostic tests. With the advancement of molecular diagnostics, these products are now capable of delivering highly accurate and faster results compared to traditional testing methods, making them indispensable in modern diagnostic labs.
The molecular diagnostics segment held a 25.5% market share in 2024 and is expected to grow significantly, reaching USD 40.8 billion by 2034. This segment benefits from ongoing innovations that enhance test accuracy, turnaround time, and ease of use. Molecular diagnostic tools are now the preferred option for detecting infectious diseases, genetic disorders, and cancer, thanks to their ability to identify pathogens and genetic mutations at a molecular level. Unlike conventional tests that often require days to return results, molecular diagnostics can produce actionable insights within hours or even minutes, enabling faster treatment decisions and better patient outcomes.
The North America In-Vitro Diagnostics Market generated USD 45.1 billion in 2024, maintaining a dominant position due to strong healthcare infrastructure, a high burden of chronic illnesses, and early adoption of advanced technologies. Despite a stringent regulatory landscape, the region continues to support the approval and commercialization of cutting-edge diagnostic solutions.
Key players in the Global In-Vitro Diagnostics Market include Abbott Laboratories, ACON Laboratories, Agilent Technologies, Becton, Dickinson and Company, Bio-Rad Laboratories, Dragerwerk, F. Hoffmann-La Roche, Danaher Corporation, Medtronic, Meridian Bioscience, Nova Biomedical, PerkinElmer, Siemens Healthineers, and Sysmex Corporation. These companies focus on research and development, strategic partnerships, and global expansion to meet the rising demand for advanced diagnostic technologies.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Base estimates and calculations
- 1.3.1 Base year calculation
- 1.3.2 Key trends for market estimation
- 1.4 Forecast model
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.5.2 Data mining sources
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Rising prevalence of infectious and chronic diseases worldwide
- 3.2.1.2 Growing adoption and rising demand for personalized medicine
- 3.2.1.3 Increasing adoption of point-of-care testing in developing countries
- 3.2.1.4 Surging number of pathology labs and services equipped with advanced diagnostics machine
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 High cost of diagnostic services
- 3.2.2.2 Unfavorable reimbursement scenario
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.5 Technological landscape
- 3.6 Future market trends
- 3.7 Gap analysis
- 3.8 Porter’s analysis
- 3.9 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.3 Company matrix analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Strategy dashboard
Chapter 5 Market Estimates and Forecast, By Product, 2021 - 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Reagents and kits
- 5.3 Instruments
Chapter 6 Market Estimates and Forecast, By Test Type, 2021 - 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Clinical chemistry
- 6.3 Immunoassay/immunochemistry
- 6.4 Molecular diagnostics
- 6.5 Hematology
- 6.6 Urinalysis
- 6.7 Other test types
Chapter 7 Market Estimates and Forecast, By Application, 2021 - 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Oncology
- 7.3 Infectious diseases
- 7.4 Diabetes
- 7.5 Cardiology
- 7.6 Nephrology
- 7.7 Autoimmune diseases
- 7.8 Drug testing/pharmacogenomics
- 7.9 Other applications
Chapter 8 Market Estimates and Forecast, By End Use, 2021 - 2034 ($ Mn)
- 8.1 Key trends
- 8.2 Hospitals
- 8.3 Diagnostic laboratories
- 8.4 Academic and research institutes
- 8.5 Other end use
Chapter 9 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Mn)
- 9.1 Key trends
- 9.2 North America
- 9.2.1 U.S.
- 9.2.2 Canada
- 9.3 Europe
- 9.3.1 Germany
- 9.3.2 UK
- 9.3.3 France
- 9.3.4 Spain
- 9.3.5 Italy
- 9.4 Asia Pacific
- 9.4.1 China
- 9.4.2 India
- 9.4.3 Japan
- 9.4.4 Australia
- 9.4.5 South Korea
- 9.5 Latin America
- 9.5.1 Brazil
- 9.5.2 Mexico
- 9.5.3 Argentina
- 9.6 Middle East and Africa
- 9.6.1 Saudi Arabia
- 9.6.2 South Africa
- 9.6.3 UAE
Chapter 10 Company Profiles
- 10.1 Abbott Laboratories
- 10.2 ACON Laboratories
- 10.3 Agilent Technologies
- 10.4 Becton, Dickinson and Company
- 10.5 Biomerieux
- 10.6 Bio-Rad Laboratories
- 10.7 Dragerwerk
- 10.8 F. Hoffmann-La Roche
- 10.9 Danaher Corporation
- 10.10 Medtronic
- 10.11 Meridian Bioscience
- 10.12 Nova Biomedical
- 10.13 PerkinElmer
- 10.14 Siemens Healthineers
- 10.15 Sysmex Corporation












