 |
市场调查报告书
商品编码
1750354
特发性血小板减少性紫斑症 (ITP) 治疗市场机会、成长动力、产业趋势分析及 2025 - 2034 年预测Idiopathic Thrombocytopenic Purpura (ITP) Therapeutics Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
2024年,全球特发性血小板减少性紫斑症 (ITP) 治疗市场规模达6.895亿美元,预计到2034年将以5.1%的复合年增长率成长,达到11亿美元。这主要得益于全球自体免疫疾病病例的增加,以及对ITP认识的提高和早期诊断的提升。诊断工具的技术进步使得早期检测ITP变得更加容易。加上许多地区医疗服务可近性的提高,有助于医疗机构及时启动治疗,进而进一步刺激治疗需求。
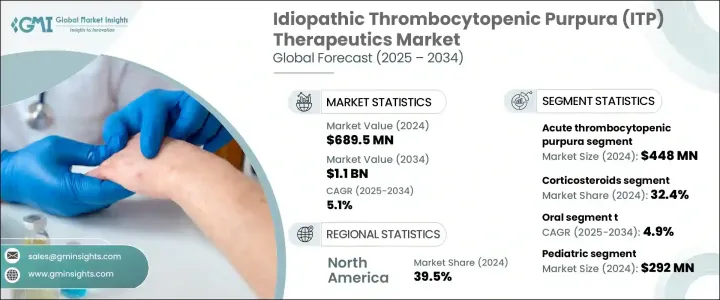
现代诊断技术,包括先进的血小板功能检测和免疫标记分析,帮助医护人员更好地理解和识别ITP。这些进展导致确诊病例显着增加,也增加了对可靠有效治疗方法的需求。治疗领域的创新,尤其是血小板生成素受体激动剂的日益普及,改善了慢性ITP患者的预后。事实证明,这些疗法比传统治疗方法更有效、更容易管理。同时,活跃的药物研发管线为治疗重症和难治性ITP带来了希望,增强了该产业的成长预期。
| 市场范围 | |
|---|---|
| 起始年份 | 2024 |
| 预测年份 | 2025-2034 |
| 起始值 | 6.895亿美元 |
| 预测值 | 11亿美元 |
| 复合年增长率 | 5.1% |
在疾病类型中,急性血小板减少性紫斑症(ITP)在2024年的市场价值为4.48亿美元,这主要得益于儿童病例数量的增加,这些病例通常在病毒感染后发展为急性ITP。这些新的紧急病例需要快速的治疗干预,主要依靠静脉注射免疫球蛋白和皮质类固醇等经济有效的治疗方法。由于这些患者的出血风险较高,立即治疗至关重要,因此对第一线治疗药物的需求持续稳定。
2024年,皮质类固醇市场占有32.4%的份额。其在抑制免疫活性和提高血小板水平方面的有效性使其成为首选。此外,价格实惠、广泛可用以及与成人和儿科治疗方案相容也使其成为市场主导。皮质类固醇因其口服和注射剂型、给药简便以及被纳入主要临床指南而经常被处方。
2024年,美国特发性血小板减少性紫斑症 (ITP) 治疗市场规模达2.486亿美元。高发病率、早期疾病检测以及专科护理机构的普及,共同推动了该市场的扩张。美国拥有成熟的医疗基础设施和完善的报销体系,确保急性和慢性ITP患者都能更广泛地获得先进的治疗方案。正在进行的临床试验和学术研究也加速了下一代疗法的开发,促进了生物製剂和血小板生成素受体激动剂的稳定应用。
该行业的主要参与者包括Sobi、Intas Pharmaceuticals、安进、诺华、Kedrion Biopharma、罗氏製药、葛兰素史克、杰富林、基立福和Octapharma AG。为了稳固立足,领先公司正在透过收购和推出新型生物製剂来扩大其产品组合。他们专注于获得FDA和EMA批准,用于治疗急性和慢性ITP的先进疗法。与研究机构的策略合作推动创新,而以患者为中心的解决方案(例如自主注射剂)的投资则增强了市场竞争力。向新兴医疗保健市场的全球扩张进一步支持了长期成长。
目录
第一章:方法论与范围
第二章:执行摘要
第三章:行业洞察
- 产业生态系统分析
- 产业衝击力
- 成长动力
- ITP盛行率上升
- 治疗方案的进展
- 提高认识和早期诊断
- 产业陷阱与挑战
- 某些疗法的不良反应
- 成长动力
- 成长潜力分析
- 监管格局
- 川普政府关税
- 对贸易的影响
- 贸易量中断
- 报復措施
- 对产业的影响
- 供应方影响(原料)
- 主要材料价格波动
- 供应链重组
- 生产成本影响
- 需求面影响(售价)
- 价格传导至终端市场
- 市占率动态
- 消费者反应模式
- 供应方影响(原料)
- 受影响的主要公司
- 策略产业反应
- 供应链重组
- 定价和产品策略
- 政策参与
- 展望与未来考虑
- 对贸易的影响
- 管道分析
- 波特的分析
- PESTEL分析
第四章:竞争格局
- 介绍
- 公司矩阵分析
- 主要市场参与者的竞争分析
- 竞争定位矩阵
- 策略仪表板
第五章:市场估计与预测:依疾病类型,2021 年至 2034 年
- 主要趋势
- 急性血小板减少性紫斑
- 慢性血小板减少性紫斑
第六章:市场估计与预测:按产品,2021 年至 2034 年
- 主要趋势
- 皮质类固醇
- 静脉注射免疫球蛋白(IVIG)
- 血小板生成素受体激动剂(TPO-RA)
- 其他产品
第七章:市场估计与预测:依管理路线,2021 年至 2034 年
- 主要趋势
- 口服
- 注射剂
第八章:市场估计与预测:按年龄组,2021 年至 2034 年
- 主要趋势
- 儿科
- 成人
- 老年
第九章:市场估计与预测:按配销通路,2021 年至 2034 年
- 主要趋势
- 医院药房
- 零售药局
- 网路药局
第十章:市场估计与预测:按地区,2021 年至 2034 年
- 主要趋势
- 北美洲
- 我们
- 加拿大
- 欧洲
- 德国
- 英国
- 法国
- 义大利
- 西班牙
- 荷兰
- 亚太地区
- 中国
- 日本
- 印度
- 澳洲
- 韩国
- 拉丁美洲
- 巴西
- 墨西哥
- 阿根廷
- 中东和非洲
- 南非
- 沙乌地阿拉伯
- 阿联酋
第 11 章:公司简介
- Amgen
- Argenx
- CSL Behring
- F. Hoffmann-La Roche
- GlaxoSmithKline
- Grifols
- Intas Pharmaceuticals
- Kedrion Biopharma
- Kissei Pharmaceutical
- Novartis
- Octapharma AG
- Rigel Pharmaceuticals
- Sobi
The Global Idiopathic Thrombocytopenic Purpura (ITP) Therapeutics Market was valued at USD 689.5 million in 2024 and is estimated to grow at a CAGR of 5.1% to reach USD 1.1 billion by 2034, driven by the rising cases of autoimmune disorders worldwide, along with improved awareness and early diagnosis of ITP. Technological advancements in diagnostic tools are making it easier to detect ITP in its early stages. This, combined with greater healthcare accessibility in many regions, is helping healthcare providers initiate prompt treatment, further boosting therapeutic demand.

Modern diagnostic techniques, including advanced platelet function assays and immune marker analysis, have helped healthcare professionals better understand and identify ITP. These developments lead to a noticeable rise in confirmed diagnoses, increasing the need for reliable and effective treatment methods. Innovations in therapeutics, particularly the growing use of thrombopoietin receptor agonists, improve outcomes for patients with chronic forms of the condition. These therapies are proving to be more effective and easier to manage compared to traditional treatment methods. Meanwhile, an active drug development pipeline offers hope for treating severe and refractory cases of ITP, reinforcing growth projections for the industry.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $689.5 Million |
| Forecast Value | $1.1 Billion |
| CAGR | 5.1% |
Among disease types, the acute thrombocytopenic purpura segment was valued at USD 448 million in 2024, driven by the increasing number of pediatric cases where acute ITP often develops following viral infections. These new and urgent cases require rapid therapeutic intervention, primarily relying on cost-effective treatments like intravenous immunoglobulin and corticosteroids. Immediate treatment is critical due to the heightened risk of bleeding in these patients, leading to a steady demand for first-line therapies.
The corticosteroids segment held a 32.4% share in 2024. Their effectiveness in suppressing immune activity and raising platelet levels makes them a preferred choice. Additionally, affordability, wide availability, and compatibility with adult and pediatric treatment protocols contribute to their dominance. Corticosteroids are frequently prescribed due to their oral and injectable forms, simple administration, and inclusion in major clinical guidelines.
U.S. Idiopathic Thrombocytopenic Purpura (ITP) Therapeutics Market generated USD 248.6 million in 2024. The combination of a high incidence rate, early disease detection, and widespread access to specialized care facilities has supported the expansion of this market. The country benefits from a mature healthcare infrastructure and well-established reimbursement systems, which ensure broader access to advanced treatment options for both acute and chronic ITP cases. Ongoing clinical trials and academic research have also accelerated the development of next-generation therapies, contributing to the steady adoption of biologics and thrombopoietin receptor agonists.
Key players in this industry include Sobi, Intas Pharmaceuticals, Amgen, Novartis, Kedrion Biopharma, F. Hoffmann-La Roche, GlaxoSmithKline, CSL Behring, Grifols, and Octapharma AG. To secure a strong foothold, leading companies are expanding their product portfolios through acquisitions and the launch of novel biologics. They focus on securing FDA and EMA approvals for advanced therapies targeting acute and chronic ITP. Strategic collaborations with research institutions drive innovation, while investments in patient-centric solutions, such as self-administered injectables, enhance market competitiveness. Global expansion into emerging healthcare markets further supports long-term growth.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definition
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Base estimates and calculations
- 1.3.1 Base year calculation
- 1.3.2 Key trends for market estimation
- 1.4 Forecast model
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.5.2 Data mining sources
Chapter 2 Executive Summary
- 2.1 Industry 360° synopsis
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Rising prevalence of ITP
- 3.2.1.2 Advancements in therapeutic options
- 3.2.1.3 Growing awareness and early diagnosis
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 Adverse effects of certain therapies
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.5 Trump administration tariffs
- 3.5.1 Impact on trade
- 3.5.1.1 Trade volume disruptions
- 3.5.1.2 Retaliatory measures
- 3.5.2 Impact on the industry
- 3.5.2.1 Supply-side impact (raw materials)
- 3.5.2.1.1 Price volatility in key materials
- 3.5.2.1.2 Supply chain restructuring
- 3.5.2.1.3 Production cost implications
- 3.5.2.2 Demand-side impact (selling price)
- 3.5.2.2.1 Price transmission to end markets
- 3.5.2.2.2 Market share dynamics
- 3.5.2.2.3 Consumer response patterns
- 3.5.2.1 Supply-side impact (raw materials)
- 3.5.3 Key companies impacted
- 3.5.4 Strategic industry responses
- 3.5.4.1 Supply chain reconfiguration
- 3.5.4.2 Pricing and product strategies
- 3.5.4.3 Policy engagement
- 3.5.5 Outlook and future considerations
- 3.5.1 Impact on trade
- 3.6 Pipeline analysis
- 3.7 Porter's analysis
- 3.8 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company matrix analysis
- 4.3 Competitive analysis of major market players
- 4.4 Competitive positioning matrix
- 4.5 Strategy dashboard
Chapter 5 Market Estimates and Forecast, By Disease Type, 2021 – 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Acute thrombocytopenic purpura
- 5.3 Chronic thrombocytopenic purpura
Chapter 6 Market Estimates and Forecast, By Product, 2021 – 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Corticosteroids
- 6.3 Intravenous immunoglobulins (IVIG)
- 6.4 Thrombopoietin receptor agonists (TPO-RA)
- 6.5 Other products
Chapter 7 Market Estimates and Forecast, By Route of Administration, 2021 – 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Oral
- 7.3 Injectable
Chapter 8 Market Estimates and Forecast, By Age Group, 2021 – 2034 ($ Mn)
- 8.1 Key trends
- 8.2 Pediatric
- 8.3 Adult
- 8.4 Geriatric
Chapter 9 Market Estimates and Forecast, By Distribution Channel, 2021 – 2034 ($ Mn)
- 9.1 Key trends
- 9.2 Hospital pharmacies
- 9.3 Retail pharmacies
- 9.4 Online pharmacies
Chapter 10 Market Estimates and Forecast, By Region, 2021 – 2034 ($ Mn)
- 10.1 Key trends
- 10.2 North America
- 10.2.1 U.S.
- 10.2.2 Canada
- 10.3 Europe
- 10.3.1 Germany
- 10.3.2 UK
- 10.3.3 France
- 10.3.4 Italy
- 10.3.5 Spain
- 10.3.6 Netherlands
- 10.4 Asia Pacific
- 10.4.1 China
- 10.4.2 Japan
- 10.4.3 India
- 10.4.4 Australia
- 10.4.5 South Korea
- 10.5 Latin America
- 10.5.1 Brazil
- 10.5.2 Mexico
- 10.5.3 Argentina
- 10.6 Middle East and Africa
- 10.6.1 South Africa
- 10.6.2 Saudi Arabia
- 10.6.3 UAE
Chapter 11 Company Profiles
- 11.1 Amgen
- 11.2 Argenx
- 11.3 CSL Behring
- 11.4 F. Hoffmann-La Roche
- 11.5 GlaxoSmithKline
- 11.6 Grifols
- 11.7 Intas Pharmaceuticals
- 11.8 Kedrion Biopharma
- 11.9 Kissei Pharmaceutical
- 11.10 Novartis
- 11.11 Octapharma AG
- 11.12 Rigel Pharmaceuticals
- 11.13 Sobi





