 |
市场调查报告书
商品编码
1750480
败血症治疗市场机会、成长动力、产业趋势分析及 2025 - 2034 年预测Sepsis Therapeutics Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
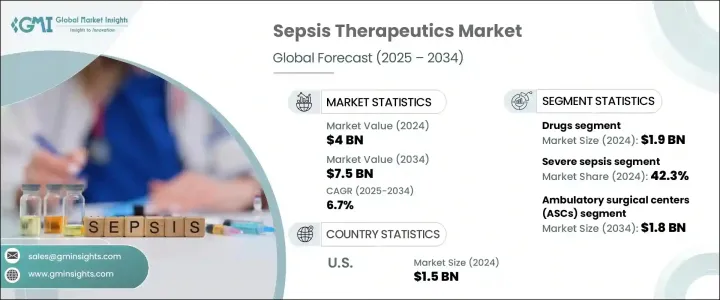
2024年,全球败血症治疗市场规模达40亿美元,预计年复合成长率将达6.7%,2034年将达75亿美元。败血症是一种危及生命的疾病,由不受控制的感染免疫反应引发,需要立即进行针对性的医疗治疗。当人体的防御系统失控时,可能会损害健康组织并导致严重的器官衰竭。随着全球医院内感染负担的不断加重以及人口老化,对下一代疗法的需求日益迫切。
全球医疗保健系统日益面临提供及时精准败血症治疗的压力,加剧了对下一代治疗方案的追求。随着败血症发病率持续上升,製药和生技公司正在加强研发投入,以期发现突破性疗法。这种创新浪潮尤其体现在不断增长的生物製剂和免疫疗法研发管线中,这些药物旨在调节人体免疫反应并提高患者存活率。由于传统抗生素因抗菌素抗药性而面临局限性,对新型药物和精准疗法的需求空前高涨。临床认知的进步,加上监管环境的支持,正在进一步加速败血症治疗策略的演变。
| 市场范围 | |
|---|---|
| 起始年份 | 2024 |
| 预测年份 | 2025-2034 |
| 起始值 | 40亿美元 |
| 预测值 | 75亿美元 |
| 复合年增长率 | 6.7% |
2024年,药品领域收入达19亿美元。抗生素、抗病毒药物、抗真菌药物、血管收缩剂和免疫调节剂仍然是败血症管理的基础。及时使用广谱抗生素对于在治疗早期对抗病原体至关重要。包括皮质类固醇和血管加压素在内的支持疗法可以稳定患者病情并减少全身性发炎。由于抗药性导致脓毒症病例日益复杂,药物开发商正致力于开发更具针对性的疗法,以期取得更佳的临床疗效。
2024年,严重败血症占比42.3%。许多病例会迅速进展至这一关键阶段,其特征是广泛的发炎和器官衰竭。这些临床表现日益复杂,尤其是在抗药性感染威胁日益加剧的背景下,这凸显了对更有效、多标靶治疗方案的需求。医疗保健提供者强调早期诊断和个人化医疗,以防止病情恶化并改善患者预后。
2024年,美国败血症治疗市场规模达15亿美元。这一强劲增长得益于多种因素,包括老龄人口增长、医院内感染率高以及医疗服务水平的持续改善。将人工智慧和机器学习融入败血症诊断领域正日益受到关注,从而能够更快、更准确地识别高风险患者。此外,积极的研发项目和对产品线拓展的高度重视巩固了美国在该领域的领先地位。这些发展正在塑造一个未来,精准驱动、技术赋能的医疗服务将在败血症管理中扮演核心角色。
在全球败血症治疗市场,各公司正采取以创新、临床合作和产品线拓展为重点的策略,以巩固其市场地位。北美和欧洲的参与者强调研究联盟,以开发先进的生物製剂、下一代抗生素和免疫疗法。辉瑞、罗氏和西普拉等公司正在投资新型给药系统和人工智慧整合诊断平台,以加快败血症的检测和介入速度。许多公司,尤其是在亚太市场,正在进行II期和III期临床试验,以挖掘新兴需求。在拉丁美洲、中东和非洲等败血症治疗缺口仍然巨大的市场,许可协议、合併和区域製造能力也日益受到关注。
目录
第一章:方法论与范围
第二章:执行摘要
第三章:行业洞察
- 产业生态系统分析
- 产业衝击力
- 成长动力
- 败血症发生率上升
- 院内感染(HAI)的盛行率不断上升
- 诊断和治疗方案的技术进步
- 产业陷阱与挑战
- 治疗费用高昂
- 严格的监管审批
- 成长动力
- 成长潜力分析
- 监管格局
- 川普政府关税
- 对贸易的影响
- 贸易量中断
- 报復措施
- 对产业的影响
- 供给侧影响(原料)
- 主要材料价格波动
- 供应链重组
- 生产成本影响
- 需求面影响(售价)
- 价格传导至终端市场
- 市占率动态
- 消费者反应模式
- 供给侧影响(原料)
- 受影响的主要公司
- 策略产业反应
- 供应链重组
- 定价和产品策略
- 政策参与
- 展望与未来考虑
- 对贸易的影响
- 波特的分析
- PESTEL分析
第四章:竞争格局
- 介绍
- 公司矩阵分析
- 主要市场参与者的竞争分析
- 竞争定位矩阵
- 策略仪表板
第五章:市场估计与预测:按治疗类型,2021 - 2034 年
- 主要趋势
- 药物
- 抗生素
- 抗病毒/抗真菌
- 其他药物
- 静脉输液
- 氧气疗法
- 手术
- 其他治疗类型
第六章:市场估计与预测:依疾病类型,2021 - 2034 年
- 主要趋势
- 轻度败血症
- 严重败血症
- 感染性休克
第七章:市场估计与预测:依最终用途,2021 - 2034 年
- 主要趋势
- 医院
- 诊所
- 门诊手术中心
- 其他最终用途
第八章:市场估计与预测:按地区,2021 - 2034 年
- 主要趋势
- 北美洲
- 我们
- 加拿大
- 欧洲
- 德国
- 英国
- 法国
- 西班牙
- 义大利
- 荷兰
- 亚太地区
- 中国
- 日本
- 印度
- 澳洲
- 韩国
- 拉丁美洲
- 巴西
- 墨西哥
- 阿根廷
- 中东和非洲
- 南非
- 沙乌地阿拉伯
- 阿联酋
第九章:公司简介
- Abbott Laboratories
- Adrenomed
- Astellas Pharma
- Aurobindo Pharmaceuticals
- Aurora St. Luke's Medical Center
- Azurity Pharmaceuticals
- Bosch Pharmaceuticals
- Christiana hospitals
- Cipla
- Drive DeVilbiss Healthcare
- F. Hoffmann La Roche
- Medical Services Company
- Pfizer
- Torrent Pharmaceuticals
- West Virginia University Hospital
The Global Sepsis Therapeutics Market was valued at USD 4 billion in 2024 and is estimated to grow at a CAGR of 6.7% to reach USD 7.5 billion by 2034, driven by a life-threatening condition triggered by an unregulated immune reaction to infection, which requires immediate and targeted medical attention. When the body's defense system spirals out of control, it can damage healthy tissues and lead to severe organ failure. With the rising global burden of hospital-acquired infections and an aging population, there is an urgent need for next-generation therapies.

Healthcare systems worldwide are increasingly strained by the need to deliver timely and precise sepsis care, intensifying the push for next-generation treatment options. As the incidence of sepsis continues to rise, pharmaceutical and biotech companies are stepping up investments in research and development to discover breakthrough therapies. This surge in innovation is especially apparent in the growing pipeline of biologics and immunotherapies that aim to modulate the body's immune response and improve patient survival rates. With traditional antibiotics facing limitations due to antimicrobial resistance, the demand for novel drug classes and precision-based therapies has never been higher. Advancements in clinical understanding, combined with supportive regulatory environments, are further accelerating the evolution of sepsis treatment strategies.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $4 Billion |
| Forecast Value | $7.5 Billion |
| CAGR | 6.7% |
In 2024, the drug segment generated USD 1.9 billion. Antibiotics, antivirals, antifungals, vasoconstrictors, and immunomodulators continue to be the foundation of sepsis management. Prompt administration of broad-spectrum antibiotics is essential to combat pathogens early in treatment. Supportive therapies, including corticosteroids and vasopressors, stabilize patients and reduce systemic inflammation. With sepsis cases becoming more complex due to drug resistance, pharmaceutical developers are focusing on more targeted therapies that can offer greater clinical outcomes.
In 2024, the severe sepsis segment held a 42.3% share. Many cases progress rapidly to this critical stage, characterized by extensive inflammation and organ failure. The increasing complexity of these clinical presentations, especially amid the growing threat of drug-resistant infections, underscores the need for more potent, multi-targeted therapeutic solutions. Healthcare providers emphasize early-stage diagnostics and personalized medicine to prevent escalation and improve patient outcomes.
United States Sepsis Therapeutics Market generated USD 1.5 billion in 2024. Several factors contribute to this robust performance, including a rising elderly population, a high rate of hospital-acquired infections, and continuous improvements in healthcare delivery. Integrating AI and machine learning in sepsis diagnostics is gaining traction, allowing for faster, more accurate identification of at-risk patients. Additionally, active R&D programs and a strong focus on expanding product pipelines reinforce the country's leadership in the field. These developments are shaping a future where precision-driven, technology-enabled care plays a central role in sepsis management.
In the Global Sepsis Therapeutics Market companies are adopting strategies focused on innovation, clinical partnerships, and pipeline expansion to solidify their position. Players in North America and Europe emphasize research alliances to develop advanced biologics, next-gen antibiotics, and immunotherapies. Firms such as Pfizer, F. Hoffmann-La Roche, and Cipla are investing in novel drug delivery systems and AI-integrated diagnostic platforms to speed up sepsis detection and intervention. Many companies are progressing through Phase II and III clinical trials, especially in the Asia Pacific market, to tap into emerging demand. Licensing agreements, mergers, and regional manufacturing capabilities are also gaining traction in markets like Latin America and the Middle East & Africa, where the sepsis treatment gap remains wide.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Base estimates and calculations
- 1.3.1 Base year calculation
- 1.3.2 Key trends for market estimation
- 1.4 Forecast model
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.5.2 Data mining sources
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Increasing incidences of sepsis
- 3.2.1.2 Growing prevalence of hospital-acquired infections (HAIs)
- 3.2.1.3 Technological advancement in diagnostics and treatment options
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 High cost of treatment
- 3.2.2.2 Stringent regulatory approvals
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.5 Trump administration tariffs
- 3.5.1 Impact on trade
- 3.5.1.1 Trade volume disruptions
- 3.5.1.2 Retaliatory measures
- 3.5.2 Impact on the Industry
- 3.5.2.1 Supply-side impact (raw materials)
- 3.5.2.1.1 Price volatility in key materials
- 3.5.2.1.2 Supply chain restructuring
- 3.5.2.1.3 Production cost implications
- 3.5.2.2 Demand-side impact (selling price)
- 3.5.2.2.1 Price transmission to end markets
- 3.5.2.2.2 Market share dynamics
- 3.5.2.2.3 Consumer response patterns
- 3.5.2.1 Supply-side impact (raw materials)
- 3.5.3 Key companies impacted
- 3.5.4 Strategic industry responses
- 3.5.4.1 Supply chain reconfiguration
- 3.5.4.2 Pricing and product strategies
- 3.5.4.3 Policy engagement
- 3.5.5 Outlook and future considerations
- 3.5.1 Impact on trade
- 3.6 Porter's analysis
- 3.7 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company matrix analysis
- 4.3 Competitive analysis of major market players
- 4.4 Competitive positioning matrix
- 4.5 Strategy dashboard
Chapter 5 Market Estimates and Forecast, By Treatment Type, 2021 - 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Drugs
- 5.2.1 Antibiotics
- 5.2.2 Anti-viral/ Anti-fungal
- 5.2.3 Other drugs
- 5.3 I.V fluids
- 5.4 Oxygen therapy
- 5.5 Surgery
- 5.6 Other treatment types
Chapter 6 Market Estimates and Forecast, By Disease Type, 2021 - 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Mild sepsis
- 6.3 Severe sepsis
- 6.4 Septic shock
Chapter 7 Market Estimates and Forecast, By End Use, 2021 - 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Hospitals
- 7.3 Clinics
- 7.4 Ambulatory surgical centers
- 7.5 Other end use
Chapter 8 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Mn)
- 8.1 Key trends
- 8.2 North America
- 8.2.1 U.S.
- 8.2.2 Canada
- 8.3 Europe
- 8.3.1 Germany
- 8.3.2 UK
- 8.3.3 France
- 8.3.4 Spain
- 8.3.5 Italy
- 8.3.6 Netherlands
- 8.4 Asia Pacific
- 8.4.1 China
- 8.4.2 Japan
- 8.4.3 India
- 8.4.4 Australia
- 8.4.5 South Korea
- 8.5 Latin America
- 8.5.1 Brazil
- 8.5.2 Mexico
- 8.5.3 Argentina
- 8.6 Middle East and Africa
- 8.6.1 South Africa
- 8.6.2 Saudi Arabia
- 8.6.3 UAE
Chapter 9 Company Profiles
- 9.1 Abbott Laboratories
- 9.2 Adrenomed
- 9.3 Astellas Pharma
- 9.4 Aurobindo Pharmaceuticals
- 9.5 Aurora St. Luke’s Medical Center
- 9.6 Azurity Pharmaceuticals
- 9.7 Bosch Pharmaceuticals
- 9.8 Christiana hospitals
- 9.9 Cipla
- 9.10 Drive DeVilbiss Healthcare
- 9.11 F. Hoffmann La Roche
- 9.12 Medical Services Company
- 9.13 Pfizer
- 9.14 Torrent Pharmaceuticals
- 9.15 West Virginia University Hospital





