|
市场调查报告书
商品编码
1750515
经导管栓塞及封堵装置市场机会、成长动力、产业趋势分析及 2025 - 2034 年预测Transcatheter Embolization And Occlusion Devices Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
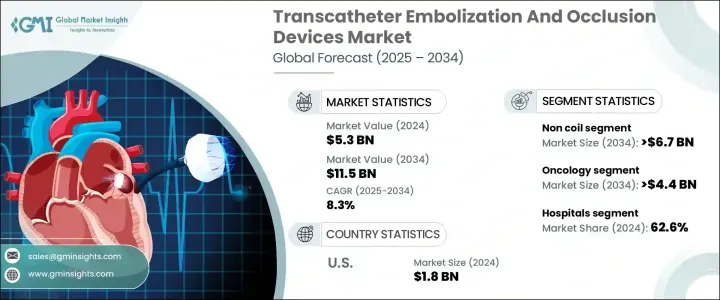
2024年,全球经导管栓塞与封堵装置市场规模达53亿美元,预计到2034年将以8.3%的复合年增长率成长,达到115亿美元。推动这一增长的因素包括癌症等慢性疾病的发生率不断上升、妊娠併发症、血管畸形以及血友病等出血性疾病,这些疾病都需要专科医疗照护。此外,全球人口老化导致人们对生活品质更高的手术需求不断增长,这些手术旨在降低手术创伤并缩短住院时间,这使得经导管栓塞与封堵装置(TEO)对医生和患者都颇具吸引力。
肝癌和肝细胞癌病例的增加,尤其是在亚太地区,导致栓塞疗法(例如经动脉化疗栓塞术 (TACE))的应用日益广泛。导管技术、医学影像和生物相容性材料的进步提高了这些手术的安全性和准确性,从而促进了其更广泛的应用。此外,人们对微创治疗方案的认识不断提高,使得子宫肌瘤栓塞术 (UFE) 成为寻求传统外科手术替代方案的女性群体的热门选择。与子宫切除术不同,UFE 可以保留子宫,缩短恢復时间,并最大程度地减少住院时间,使其成为更具吸引力且更方便患者的治疗方案。病患偏好的这种转变促使更多妇科医师和介入放射科医师推荐栓塞技术,进而推动了经导管栓塞装置的普及。
| 市场范围 | |
|---|---|
| 起始年份 | 2024 |
| 预测年份 | 2025-2034 |
| 起始值 | 53亿美元 |
| 预测值 | 115亿美元 |
| 复合年增长率 | 8.3% |
市场按设备类型细分为非线圈和线圈两类。非线圈市场预计将以 8.6% 的复合年增长率成长,到 2034 年达到 67 亿美元,这得益于微球和液体栓塞剂等新型栓塞剂的普及,以及相比传统线圈设备在闭塞过程中控制性能更佳的栓塞。非线圈解决方案尤其适用于治疗肝癌、动静脉畸形、胃肠道出血和子宫肌瘤的标靶栓塞手术。它们能够导航复杂的血管解剖结构并获得一致的结果,因此越来越受到介入放射科医师的青睐。
在应用方面,肿瘤领域预计将推动业务成长,复合年增长率达8.8%,到2034年将达到44亿美元。这得归功于全球癌症负担的持续加重,尤其是肝癌、肾癌和肺癌,这些癌症通常需要更局部化、侵入性更低的治疗方案。经动脉化疗栓塞术(TACE)和放射栓塞术作为重要的介入性肿瘤治疗手段的应用显着增长。肝细胞癌病例的不断增加,主要集中在亚太和拉丁美洲地区,也见证了栓塞疗法的应用日益增加。
美国经导管栓塞和封堵装置市场在2024年达到18亿美元,预计2025年至2034年期间的复合年增长率将达到7.4%。栓塞治疗装置的需求在很大程度上受到癌症、胃肠道出血、动脉瘤和子宫肌瘤等广泛健康问题的影响,而微创栓塞疗法是治疗这些疾病的最佳方法。美国占主导地位的医疗保健支出及其完善的医疗基础设施促进了新型经导管栓塞术(TEO)的推广。此外,领先市场製造商的不断涌现以及美国食品药物管理局(FDA)对新型高效栓塞剂和输送系统的批准激增,也推动了市场的成长。
全球经导管栓塞和封堵装置行业的知名企业包括雅培、Acandis、Balt、波士顿科学、COOK MEDICAL、Edwards Lifesciences、强生、LEPU MEDICAL、美敦力、Merit Medical、MicroVention、Penumbra、Shape Memory Medical、SIRTEX、Stryker 和 TERUMO。为了巩固市场地位,经导管栓塞和封堵装置市场的公司正专注于几项关键策略。这些策略包括投资研发,以创造满足医疗服务提供者和患者不断变化的需求的创新产品。公司正在与医院和医疗机构建立策略伙伴关係和合作关係,以提高产品采用率并扩大市场范围。此外,该公司还致力于扩展其产品组合,以满足更广泛的医疗条件和应用需求。
目录
第一章:方法论与范围
第二章:执行摘要
第三章:行业洞察
- 产业生态系统分析
- 产业衝击力
- 成长动力
- 转向微创手术
- 提高介入放射学能力
- 慢性病盛行率上升
- 栓塞材料的技术进步
- 创伤发生率增加
- 产业陷阱与挑战
- 设备和手术成本高昂
- 非标靶栓塞的风险
- 成长动力
- 成长潜力分析
- 监管格局
- 川普政府关税
- 对贸易的影响
- 贸易量中断
- 各国应对措施
- 对产业的影响
- 供应方影响(製造成本)
- 主要材料价格波动
- 供应链重组
- 生产成本影响
- 需求面影响(消费者成本)
- 价格传导至终端市场
- 市占率动态
- 消费者反应模式
- 供应方影响(製造成本)
- 受影响的主要公司
- 策略产业反应
- 供应链重组
- 定价和产品策略
- 政策参与
- 展望与未来考虑
- 对贸易的影响
- 技术格局
- 差距分析
- 波特的分析
- PESTEL分析
- 价值链分析
第四章:竞争格局
- 介绍
- 公司矩阵分析
- 公司市占率分析
- 主要市场参与者的竞争分析
- 竞争定位矩阵
- 策略仪表板
第五章:市场估计与预测:按设备类型,2021 - 2034 年
- 主要趋势
- 无线圈
- 流量转向装置
- 栓塞颗粒
- 液态栓塞剂
- 其他非线圈设备类型
- 线圈
- 可推式线圈
- 可拆卸弹簧
第六章:市场估计与预测:按应用,2021 - 2034 年
- 主要趋势
- 肿瘤学
- 週边血管疾病
- 神经病学
- 泌尿科
- 其他应用
第七章:市场估计与预测:依最终用途,2021 - 2034 年
- 主要趋势
- 医院
- 门诊手术中心
- 其他最终用途
第八章:市场估计与预测:按地区,2021 - 2034 年
- 主要趋势
- 北美洲
- 我们
- 加拿大
- 欧洲
- 德国
- 英国
- 法国
- 西班牙
- 义大利
- 荷兰
- 亚太地区
- 中国
- 日本
- 印度
- 澳洲
- 韩国
- 拉丁美洲
- 巴西
- 墨西哥
- 阿根廷
- 中东和非洲
- 南非
- 沙乌地阿拉伯
- 阿联酋
第九章:公司简介
- Abbott
- acandis
- balt
- Boston Scientific
- COOK MEDICAL
- Edwards Lifesciences
- Johnson & Johnson
- LEPU MEDICAL
- Medtronic
- Merit Medical
- MicroVention
- Penumbra
- shape memory medical
- SIRTEX
- stryker
- TERUMO
The Global Transcatheter Embolization And Occlusion Devices Market was valued at USD 5.3 billion in 2024 and is estimated to grow at a CAGR of 8.3% to reach USD 11.5 billion by 2034, driven by several factors, including the increasing prevalence of chronic diseases such as cancer, complicated pregnancies, vascular malformations, and hemorrhagic disorders like hemophilia, all of which require specialized medical attention. Additionally, the aging global population is leading to a higher demand for quality-of-life procedures that reduce the invasiveness of surgery and shorten hospital stays, making TEO devices favorable to both physicians and patients.

The rise in cases of liver cancer and hepatocellular carcinoma, particularly in the Asia Pacific region, has led to an increased use of embolization therapies like transarterial chemoembolization (TACE). Advancements in catheter technology, medical imaging, and biocompatible materials have improved the safety and accuracy of these procedures, encouraging more widespread adoption. Additionally, the rising awareness about minimally invasive treatment options has made uterine fibroid embolization (UFE) a popular choice among women seeking alternatives to traditional surgical procedures. Unlike hysterectomy, UFE preserves the uterus, reduces recovery time, and minimizes hospital stays, making it a more attractive and patient-friendly solution. This shift in patient preference is encouraging more gynecologists and interventional radiologists to recommend embolization techniques, which is, in turn, boosting the adoption of transcatheter embolization devices.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $5.3 Billion |
| Forecast Value | $11.5 Billion |
| CAGR | 8.3% |
The market is segmented by device type into non-coil and coil categories. The non-coil segment is expected to grow at a CAGR of 8.6%, reaching USD 6.7 billion by 2034, attributed to the adoption of new embolic agents like microspheres and liquid embolics, along with plugs that offer superior control during occlusion compared to traditional coil-based devices. Non-coil solutions are particularly preferred in targeted embolization procedures used in treating liver cancer, arteriovenous malformations, gastrointestinal bleeding, and uterine fibroids. Their ability to navigate complex vascular anatomies with consistent results has made them increasingly favored by interventional radiologists.
In terms of application, the oncology segment is expected to drive business growth, expanding at a CAGR of 8.8%, reaching USD 4.4 billion by 2034, fueled by the increasing global burden of cancer, especially liver, kidney, and lung cancers, which often require more localized and less invasive treatment options. There has been significant growth in the adoption of transarterial chemoembolization (TACE) and radioembolization as vital interventional oncology procedures. The growing caseload of hepatocellular carcinoma, predominantly in the Asia Pacific and Latin America regions, is witnessing greater use of embolization therapies.
U.S. Transcatheter Embolization And Occlusion Devices Market accounted for USD 1.8 billion in 2024 and is anticipated to grow at a CAGR of 7.4% between 2025 to 2034. Demand for embolization therapeutic devices is greatly influenced by widespread health issues such as cancer, gastrointestinal bleeding, aneurysms, and uterine fibroids, which are optimally treated using minimally invasive embolization therapies. The country's dominant healthcare spending, along with its sophisticated healthcare infrastructure, facilitates the adoption of new TEO procedures. Furthermore, the increasing availability of leading market manufacturers and a surge in FDA approvals for new and efficient embolic agents and delivery systems fuel market growth.
Prominent players operating in the Global Transcatheter Embolization And Occlusion Devices Industry include Abbott, Acandis, Balt, Boston Scientific, COOK MEDICAL, Edwards Lifesciences, Johnson & Johnson, LEPU MEDICAL, Medtronic, Merit Medical, MicroVention, Penumbra, Shape Memory Medical, SIRTEX, Stryker, and TERUMO. To strengthen their market position, companies in the transcatheter embolization and occlusion devices market are focusing on several key strategies. These include investing in research and development to create innovative products that meet the evolving needs of healthcare providers and patients. Strategic partnerships and collaborations with hospitals and healthcare institutions are being pursued to enhance product adoption and expand market reach. Additionally, companies are focusing on expanding their product portfolios to cater to a wider range of medical conditions and applications.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Base estimates and calculations
- 1.3.1 Base year calculation
- 1.3.2 Key trends for market estimation
- 1.4 Forecast model
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.5.2 Data mining sources
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Shift toward minimally invasive procedures
- 3.2.1.2 Increasing interventional radiology capabilities
- 3.2.1.3 Rising prevalence of chronic diseases
- 3.2.1.4 Technological advancements in embolic materials
- 3.2.1.5 Increased incidence of traumatic injuries
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 High cost of devices and procedures
- 3.2.2.2 Risk of non-target embolization
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.5 Trump administration tariffs
- 3.5.1 Impact on trade
- 3.5.1.1 Trade volume disruptions
- 3.5.1.2 Country-wise Response
- 3.5.2 Impact on the industry
- 3.5.2.1 Supply-side impact (Cost of manufacturing)
- 3.5.2.1.1 Price volatility in key materials
- 3.5.2.1.2 Supply chain restructuring
- 3.5.2.1.3 Production cost implications
- 3.5.2.2 Demand-side impact (Cost to consumers)
- 3.5.2.2.1 Price transmission to end markets
- 3.5.2.2.2 Market share dynamics
- 3.5.2.2.3 Consumer response patterns
- 3.5.2.1 Supply-side impact (Cost of manufacturing)
- 3.5.3 Key companies impacted
- 3.5.4 Strategic industry responses
- 3.5.4.1 Supply chain reconfiguration
- 3.5.4.2 Pricing and product strategies
- 3.5.4.3 Policy engagement
- 3.5.5 Outlook and future considerations
- 3.5.1 Impact on trade
- 3.6 Technology landscape
- 3.7 Gap analysis
- 3.8 Porter's analysis
- 3.9 PESTEL analysis
- 3.10 Value chain analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company matrix analysis
- 4.3 Company market share analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Strategy dashboard
Chapter 5 Market Estimates and Forecast, By Device Type, 2021 - 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Non coil
- 5.2.1 Flow diverting devices
- 5.2.2 Embolization particles
- 5.2.3 Liquid embolics
- 5.2.4 Other non-coil device types
- 5.3 Coils
- 5.3.1 Pushable coils
- 5.3.2 Detachable coils
Chapter 6 Market Estimates and Forecast, By Application, 2021 - 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Oncology
- 6.3 Peripheral vascular disease
- 6.4 Neurology
- 6.5 Urology
- 6.6 Other applications
Chapter 7 Market Estimates and Forecast, By End Use, 2021 - 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Hospitals
- 7.3 Ambulatory surgical centers
- 7.4 Other end use
Chapter 8 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Mn)
- 8.1 Key trends
- 8.2 North America
- 8.2.1 U.S.
- 8.2.2 Canada
- 8.3 Europe
- 8.3.1 Germany
- 8.3.2 UK
- 8.3.3 France
- 8.3.4 Spain
- 8.3.5 Italy
- 8.3.6 Netherlands
- 8.4 Asia Pacific
- 8.4.1 China
- 8.4.2 Japan
- 8.4.3 India
- 8.4.4 Australia
- 8.4.5 South Korea
- 8.5 Latin America
- 8.5.1 Brazil
- 8.5.2 Mexico
- 8.5.3 Argentina
- 8.6 Middle East and Africa
- 8.6.1 South Africa
- 8.6.2 Saudi Arabia
- 8.6.3 UAE
Chapter 9 Company Profiles
- 9.1 Abbott
- 9.2 acandis
- 9.3 balt
- 9.4 Boston Scientific
- 9.5 COOK MEDICAL
- 9.6 Edwards Lifesciences
- 9.7 Johnson & Johnson
- 9.8 LEPU MEDICAL
- 9.9 Medtronic
- 9.10 Merit Medical
- 9.11 MicroVention
- 9.12 Penumbra
- 9.13 shape memory medical
- 9.14 SIRTEX
- 9.15 stryker
- 9.16 TERUMO