 |
市场调查报告书
商品编码
1755259
一次性十二指肠镜市场机会、成长动力、产业趋势分析及 2025 - 2034 年预测Single-use Duodenoscope Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
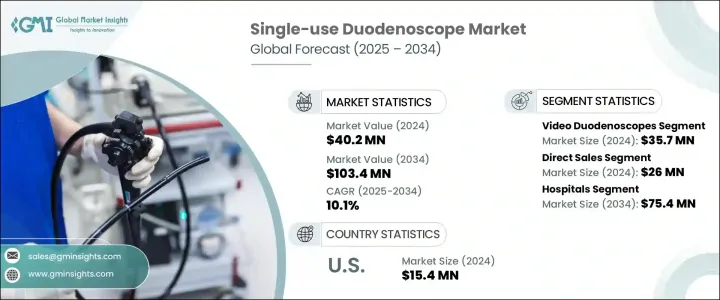
2024年,全球一次性十二指肠镜市场规模达4,020万美元,预计复合年增长率为10.1%,到2034年将达到1.034亿美元。这一增长主要源于感染控制需求的不断增长、设备设计的技术进步以及胃肠道疾病的日益增加。医疗保健机构正转向使用一次性十二指肠镜来提高病患安全性并降低交叉污染的风险,而交叉污染问题在临床环境中日益受到关注。
随着越来越多的医院和诊所采用先进的内视镜解决方案,这些设备的使用频率也越来越高,尤其是考虑到其一次性使用的特点,无需复杂的消毒程序,降低了感染风险。成像相容性的提升、更优的材料选择以及以患者为中心的设计也加速了这些一次性工具的普及。随着医疗保健产业越来越重视减少医院内感染,向一次性解决方案的过渡正成为当务之急。
| 市场范围 | |
|---|---|
| 起始年份 | 2024 |
| 预测年份 | 2025-2034 |
| 起始值 | 4020万美元 |
| 预测值 | 1.034亿美元 |
| 复合年增长率 | 10.1% |
持续的产品创新进一步推动了市场的成长,旨在提高诊断准确性和临床疗效。一次性十二指肠镜专为十二指肠内视镜手术而设计,比可重复使用型产品更安全。这些设备使用后即可丢弃,避免了任何与重复处理相关的问题。近期的设计改进使其能够更轻鬆地与现有成像平台集成,在手术过程中提供更清晰、即时的视觉效果,从而实现更精准的诊断。医疗保健专业人员更青睐一次性十二指肠镜,因为它们降低了手术复杂性,并消除了重复使用型内视镜重复处理带来的污染风险。更高的可靠性和易用性使这些设备成为内视镜手术不可或缺的一部分。
2024年,影片十二指肠镜市场规模达3,570万美元。这类镜组因其与院内成像系统配合使用时可提供高清影像而广受欢迎。其实即时成像功能使临床医生能够更清晰地发现胃肠道中即使是微小的异常。除了提高诊断准确性外,这些镜组还省去了清洁和消毒环节,从而降低了感染的可能性,而清洁和消毒环节一直以来都存在污染风险。随着医疗保健系统采用包括高清和超高清格式在内的先进视讯技术,一次性视讯十二指肠镜的需求预计将进一步增长。它们能够提供无菌、可靠的诊断工具,是安全内视镜检查程序的关键。
直销业务在2024年贡献了2,600万美元,预计到2034年将以10.5%的复合年增长率成长。直销管道使製造商能够与医疗保健专业人士建立更紧密的关係,提供客製化支援、即时回馈和客製化培训。这种协作方式能够更好地理解最终用户的需求,并加快技术支援和产品改进的交付。对于十二指肠镜等医疗器材而言,直接互动可以确保顾客服务的精准性,并增强品牌忠诚度。它还能使公司获得即时的临床回馈,调整产品策略,并根据市场需求优化未来的产品设计。
美国一次性十二指肠镜市场规模在2024年达到1,540万美元,预计2025年至2034年期间的复合年增长率将达到9.3%。由于良好的报销趋势以及对降低感染风险的日益重视,该市场需求正在不断增长。该地区的医疗保健提供者正在从可重复使用的器械转向支援感染控制工作的一次性器械。胆管阻塞、胰臟炎和胃肠道癌症等胃肠道疾病负担日益加重,对安全有效的诊断工具的需求也日益旺盛。加上政策支援和设备技术的进步,美国将继续推动整体市场扩张。
引领全球一次性十二指肠镜市场的知名公司包括宾得医疗、富士胶片、波士顿科学、安布、史托斯、奥林巴斯和奥华。这些公司正积极推行策略性倡议,以巩固其在不断成长的市场中的地位。他们专注于持续的产品升级,整合高清视讯技术,并推出更符合人体工学且更易于使用的型号。许多公司正在扩大直销队伍,以增强客户参与度和售后服务。此外,与医疗机构的合作研发和策略合作伙伴关係有助于这些公司始终领先于技术需求和临床需求。简化製造流程、获得监管部门批准以及扩大生产能力是他们抢占更大市场份额的策略。
目录
第一章:方法论与范围
第二章:执行摘要
第三章:行业洞察
- 产业生态系统分析
- 产业衝击力
- 成长动力
- 胃肠道疾病盛行率不断上升
- 技术进步
- 提高患者和医疗保健专业人员的意识
- 产业陷阱与挑战
- 与设备相关的高成本
- 成长动力
- 成长潜力分析
- 监管格局
- 我们
- 欧洲
- 技术格局
- 报销场景
- 波特的分析
- PESTEL分析
- 差距分析
- 价值链分析
- 专利分析
第四章:竞争格局
- 介绍
- 公司矩阵分析
- 公司市占率分析
- 主要市场参与者的竞争分析
- 竞争定位矩阵
- 策略仪表板
第五章:市场估计与预测:按类别,2021 - 2034 年
- 主要趋势
- 影片十二指肠镜
- 光纤十二指肠镜
第六章:市场估计与预测:按销售管道,2021 - 2034 年
- 主要趋势
- 直销
- 间接销售
第七章:市场估计与预测:依最终用途,2021 - 2034 年
- 主要趋势
- 医院
- 门诊手术中心
- 其他最终用户
第八章:市场估计与预测:按地区,2021 - 2034 年
- 主要趋势
- 北美洲
- 我们
- 加拿大
- 欧洲
- 德国
- 英国
- 法国
- 西班牙
- 义大利
- 荷兰
- 亚太地区
- 中国
- 日本
- 印度
- 澳洲
- 韩国
- 拉丁美洲
- 巴西
- 墨西哥
- 阿根廷
- 中东和非洲
- 南非
- 沙乌地阿拉伯
- 阿联酋
第九章:公司简介
- Ambu
- AOHUA
- Boston Scientific
- FUJIFILM
- OLYMPUS
- PENTAX MEDICAL
- STORZ
The Global Single-Use Duodenoscope Market was valued at USD 40.2 million in 2024 and is estimated to grow at a CAGR of 10.1% to reach USD 103.4 million by 2034. The growth is driven by the rising demand for infection control, technological improvements in device design, and the increasing prevalence of gastrointestinal conditions. Healthcare providers are turning to disposable duodenoscopes to improve patient safety and reduce the risk of cross-contamination, a concern that continues to gain traction across clinical settings.

As more hospitals and clinics adopt advanced endoscopic solutions, these devices are being used more frequently, especially in light of their one-time use format, which eliminates the need for complex sterilization procedures and reduces infection risks. Improvements in imaging compatibility, better material choices, and patient-centric designs are also accelerating the adoption of these disposable tools. With the healthcare sector increasingly focused on reducing hospital-acquired infections, the transition to single-use solutions is becoming a top priority.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $40.2 Million |
| Forecast Value | $103.4 Million |
| CAGR | 10.1% |
The market's growth is further supported by continuous product innovation to improve diagnostic accuracy and clinical outcomes. Single-use duodenoscopes are specifically engineered for endoscopic procedures in the duodenum, offering a safer alternative to reusable models. Once used, these devices are discarded, eliminating any reprocessing-related issues. Recent enhancements in design have enabled easier integration with existing imaging platforms, delivering sharper, real-time visuals during procedures and resulting in more precise diagnoses. Healthcare professionals prefer disposable models as they reduce procedural complexity and eliminate contamination risks associated with reprocessing reusable scopes. Greater reliability and ease of use make these devices an integral part of endoscopic procedures.
The video duodenoscope segment generated USD 35.7 million in 2024. These scopes have gained wide acceptance because they deliver high-definition visuals when paired with in-house imaging systems. Their real-time imaging capabilities allow clinicians to spot even minor anomalies in the gastrointestinal tract with enhanced clarity. In addition to improving diagnostic accuracy, these video scopes reduce the likelihood of infection by eliminating the cleaning and disinfection process, which has historically posed contamination risks. As healthcare systems adopt advanced video technologies, including HD and UHD formats, the demand for disposable video duodenoscopes is projected to rise further. Their ability to provide a sterile, reliable diagnostic tool makes them essential for safe endoscopic procedures.
The direct sales segment contributed USD 26 million in 2024 and is anticipated to grow at a CAGR of 10.5% through 2034. The direct sales channel enables manufacturers to build closer relationships with healthcare professionals, offering tailored support, real-time feedback, and customized training. This collaborative approach ensures a better understanding of end-user requirements and accelerates the delivery of technical support and product improvements. For medical devices like duodenoscopes, direct engagement ensures precision in customer service and strengthens brand loyalty. It also allows companies to receive immediate clinical feedback, adapt product strategies, and optimize future product designs based on market needs.
United States Single-Use Duodenoscope Market reached USD 15.4 million in 2024 and is projected to grow at a CAGR of 9.3% between 2025 and 2034. The demand is rising due to favorable reimbursement trends and a growing emphasis on minimizing infection risks. Healthcare providers in the region are shifting away from reusable options toward disposable alternatives that support infection control efforts. The increasing burden of gastrointestinal diseases, including bile duct blockages, pancreatitis, and GI cancers, is creating a strong demand for safe and effective diagnostic tools. Coupled with policy support and advancements in device technology, the U.S. continues to contribute to overall market expansion.
Prominent companies leading the Global Single-Use Duodenoscope Market include PENTAX MEDICAL, FUJIFILM, Boston Scientific, Ambu, STORZ, OLYMPUS, and AOHUA. These companies are actively pursuing strategic moves to strengthen their position in the growing market. They focus on continuous product upgrades, integrating high-definition video technologies, and launching models with improved ergonomics and ease of use. Many are expanding their direct sales force to enhance customer engagement and post-sale service. In addition, collaborative R&D efforts and strategic partnerships with healthcare facilities help these players stay ahead of technological demands and clinical needs. Streamlining manufacturing processes, securing regulatory approvals, and scaling production capabilities form their strategy to capture greater market share.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Base estimates and calculations
- 1.3.1 Base year calculation
- 1.3.2 Key trends for market estimation
- 1.4 Forecast model
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.5.2 Data mining sources
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Increasing prevalence of gastrointestinal disorders
- 3.2.1.2 Technological advancements
- 3.2.1.3 Rising awareness among patients and healthcare professionals
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 High cost associated with device
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.4.1 U.S.
- 3.4.2 Europe
- 3.5 Technology landscape
- 3.6 Reimbursement scenario
- 3.7 Porter's analysis
- 3.8 PESTEL analysis
- 3.9 Gap analysis
- 3.10 Value chain analysis
- 3.11 Patent Analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company matrix analysis
- 4.3 Company market share analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Strategy dashboard
Chapter 5 Market Estimates and Forecast, By Category, 2021 - 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Video duodenoscopes
- 5.3 Fiber optic duodenoscopes
Chapter 6 Market Estimates and Forecast, By Sales Channel, 2021 - 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Direct sales
- 6.3 In-direct sales
Chapter 7 Market Estimates and Forecast, By End Use, 2021 - 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Hospitals
- 7.3 Ambulatory surgical centers
- 7.4 Other end users
Chapter 8 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Mn)
- 8.1 Key trends
- 8.2 North America
- 8.2.1 U.S.
- 8.2.2 Canada
- 8.3 Europe
- 8.3.1 Germany
- 8.3.2 UK
- 8.3.3 France
- 8.3.4 Spain
- 8.3.5 Italy
- 8.3.6 Netherlands
- 8.4 Asia Pacific
- 8.4.1 China
- 8.4.2 Japan
- 8.4.3 India
- 8.4.4 Australia
- 8.4.5 South Korea
- 8.5 Latin America
- 8.5.1 Brazil
- 8.5.2 Mexico
- 8.5.3 Argentina
- 8.6 Middle East and Africa
- 8.6.1 South Africa
- 8.6.2 Saudi Arabia
- 8.6.3 UAE
Chapter 9 Company Profiles
- 9.1 Ambu
- 9.2 AOHUA
- 9.3 Boston Scientific
- 9.4 FUJIFILM
- 9.5 OLYMPUS
- 9.6 PENTAX MEDICAL
- 9.7 STORZ













