 |
市场调查报告书
商品编码
1773325
可吸入生物製剂市场机会、成长动力、产业趋势分析及 2025 - 2034 年预测Inhalable Biologics Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
2024年,全球可吸入生物製剂市场规模达38亿美元,预计到2034年将以16.7%的复合年增长率成长,达到174亿美元。这一增长主要源自于慢性和急性呼吸道疾病发生率的上升,包括气喘、囊性纤维化、慢性阻塞性肺病(COPD)和肺部感染。污染加剧、全球人口老化、烟草使用和遗传易感性等因素加速了这些疾病的流行,加剧了对有效治疗方案的需求。美国食品药物管理局(FDA)和欧洲药品管理局(EMA)等监管机构正积极支持可吸入性生物製剂的开发和审批,以满足这些尚未满足的医疗需求。
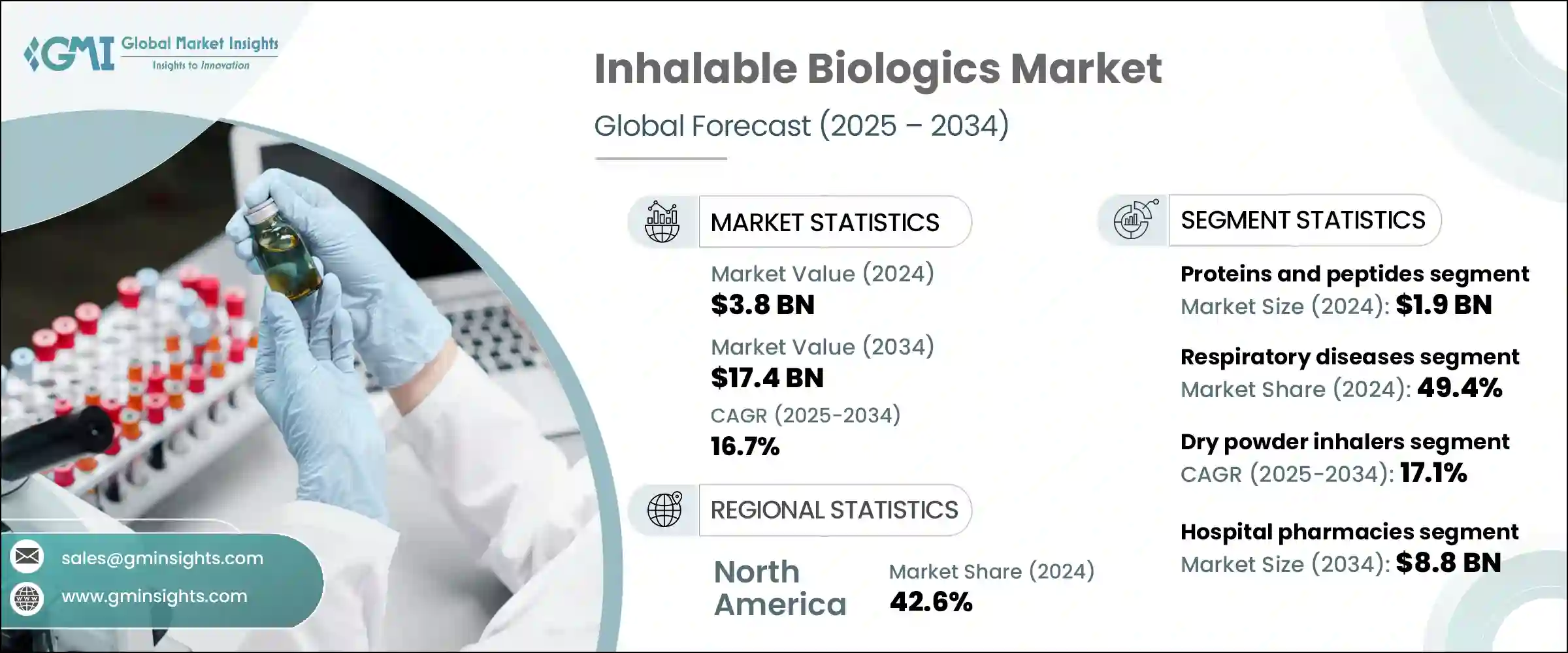
政府措施、公共部门与生物製药公司之间的合作以及对研发的大量投入,透过提高这些疗法的可及性和可负担性,进一步促进了市场成长。所有这些因素共同为这个充满活力的市场带来了巨大的机会。可吸入生物製剂涵盖了专为呼吸系统给药而设计的生物製剂的研发、生产和给药。这些疗法透过各种设备给药,例如干粉吸入器 (DPI)、雾化器、软雾吸入器和定量吸入器 (MDI)。
| 市场范围 | |
|---|---|
| 起始年份 | 2024 |
| 预测年份 | 2025-2034 |
| 起始值 | 38亿美元 |
| 预测值 | 174亿美元 |
| 复合年增长率 | 16.7% |
2024年,蛋白质和胜肽类药物市场领先,估值达19亿美元。这些生物分子因其高效力、特异性和吸入给药时的生物有效性而备受青睐,为传统注射提供了一种非侵入性替代方案。快速起效和局部治疗能力使其在治疗一系列慢性疾病(尤其是呼吸系统疾病和代谢性疾病)方面极具吸引力。它们在治疗慢性阻塞性肺病、气喘和囊性纤维化等疾病的疗效持续推动了市场需求。此外,呼吸系统疾病的日益普及也持续推动了对可吸入蛋白质和胜肽类药物的需求。
2024年,干粉吸入器细分市场的复合年增长率为17.1%。干粉吸入器(DPI)凭藉其卓越的稳定性,占据了显着的市场份额。生物製剂可在干燥状态下配製,无需像液体製剂那样进行冷链储存。这项特性使得干粉吸入器对于以全球分销为目标的製造商尤其有价值,包括冷藏基础设施有限的地区。与雾化器和计量吸入器(MDI)相比,干粉吸入器还提供了一种便捷、便携且用户友好的选择,从而提高了患者的依从性,尤其是在慢性病的长期治疗中。干粉吸入器能够将治疗性生物製剂(例如单株抗体、疫苗和胜肽)输送至肺部深处,从而增强了其治疗效果,并支持其广泛应用。
美国可吸入生物製剂市场规模在2024年达到14亿美元,并以16.7%的复合年增长率稳定成长。美国日益严重的污染水平导致气喘、过敏和鼻塞等呼吸系统疾病的发生率上升。消费者对空气污染对呼吸系统健康的有害影响的认识不断提高,这推动了对可吸入生物製剂作为预防和治疗选择的需求。美国食品药物管理局(FDA)透过监管框架和激励计画(例如孤儿药资格认定)鼓励可吸入生物製剂的开发和审批,发挥关键作用。这种监管环境,加上不断增长的患者需求和创新,正在推动美国市场的成长。
全球吸入式生物製剂市场的领导者包括阿斯特捷利康、Mannkind、United Therapeutics、Chiesi Pharmaceuticals、Merxin、勃林格殷格翰、Kamada Pharmaceuticals、Teva Pharmaceutical、艾伯维、康希诺生物和F-Hoffman Roche。这些关键企业持续推动全球创新和市场扩张。为了巩固和提升市场地位,吸入式生物製剂领域的企业着重持续研发,致力于改善药物传递技术和生物製剂製剂的稳定性。他们投资下一代吸入器设备,以最大限度地提高药物沉积效率和患者依从性,同时最大限度地减少副作用。与监管机构、研究机构和医疗保健提供者的策略合作与伙伴关係有助于加快产品审批并扩大市场范围。
目录
第一章:方法论与范围
第二章:执行摘要
第三章:行业洞察
- 产业生态系统分析
- 供应商格局
- 每个阶段的增值
- 影响价值链的因素
- 产业衝击力
- 成长动力
- 呼吸系统疾病盛行率上升
- 非侵入性给药方法越来越受欢迎
- 家庭和自我治疗的需求不断增长
- 吸入装置和生物製剂的技术进步
- 产业陷阱与挑战
- 某些可吸入生物製剂的稳定性和保存期限有限
- 开发和生产成本高
- 市场机会
- 新兴市场对本地製造业的投资不断增加
- 公私合作,提高可及性和可负担性
- 成长动力
- 成长潜力分析
- 监管格局
- 管道分析
- 未来市场趋势
- 技术格局
- 当前的技术趋势
- 新兴技术
- 波特的分析
- PESTEL分析
第四章:竞争格局
- 介绍
- 公司市占率分析
- 公司矩阵分析
- 主要市场参与者的竞争分析
- 竞争定位矩阵
- 关键进展
- 併购
- 伙伴关係与协作
- 新产品发布
- 扩张计划
第五章:市场估计与预测:依产品类型,2021 年至 2034 年
- 主要趋势
- 蛋白质和胜肽
- 疫苗
- 单株抗体
第六章:市场估计与预测:按应用,2021 年至 2034 年
- 主要趋势
- 呼吸系统疾病
- 传染病
- 糖尿病
第七章:市场估计与预测:按剂型,2021 年至 2034 年
- 主要趋势
- 干粉吸入器
- 定量吸入器
- 雾化器
- 软雾吸入器
第八章:市场估计与预测:按配销通路,2021 年至 2034 年
- 主要趋势
- 医院药房
- 零售药局
- 网路药局
第九章:市场估计与预测:按地区,2021 年至 2034 年
- 主要趋势
- 北美洲
- 我们
- 加拿大
- 欧洲
- 德国
- 英国
- 法国
- 西班牙
- 义大利
- 荷兰
- 亚太地区
- 中国
- 日本
- 印度
- 澳洲
- 韩国
- 拉丁美洲
- 巴西
- 墨西哥
- 阿根廷
- 中东和非洲
- 南非
- 沙乌地阿拉伯
- 阿联酋
第十章:公司简介
- AbbVie
- AstraZeneca
- Boehringer Ingelheim
- CanSino Biologics
- Chiesi Pharmaceuticals
- F-Hoffman Roche
- Kamada Pharmaceuticals
- Mannkind
- Merxin
- Teva Pharmaceutical
- United Therapeutics
The Global Inhalable Biologics Market was valued at USD 3.8 billion in 2024 and is estimated to grow at a CAGR of 16.7% to reach USD 17.4 billion by 2034. This growth is largely driven by the rising incidence of both chronic and acute respiratory illnesses, including asthma, cystic fibrosis, chronic obstructive pulmonary disease (COPD), and pulmonary infections. Factors such as increasing pollution levels, an aging global population, tobacco use, and genetic predispositions are accelerating the prevalence of these conditions, thereby intensifying the need for effective therapeutic options. Regulatory bodies like the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) are actively supporting the development and approval of inhalable biologics to meet these unmet medical needs.

Government initiatives, partnerships between public sectors and biopharmaceutical companies, and substantial investments in research and development are further encouraging market growth by improving the accessibility and affordability of these treatments. Collectively, these elements are paving the way for significant opportunities within this dynamic market. Inhalable biologics encompass the creation, manufacturing, and delivery of biologic therapies specifically designed for respiratory administration. These therapies are delivered through a variety of devices such as dry powder inhalers (DPIs), nebulizers, soft mist inhalers, and metered dose inhalers (MDIs).
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $3.8 Billion |
| Forecast Value | $17.4 Billion |
| CAGR | 16.7% |
The proteins and peptides segment led the market in 2024, with a valuation of USD 1.9 billion. These biological molecules are favored for their high potency, specificity, and biological effectiveness when administered via inhalation, offering a non-invasive alternative to traditional injections. The rapid therapeutic onset and ability to provide localized treatment make proteins and peptides highly attractive for managing a range of chronic illnesses, particularly respiratory and metabolic disorders. Their efficacy in treating conditions like COPD, asthma, and cystic fibrosis has driven consistent demand. Additionally, the growing prevalence of respiratory ailments continues to fuel the need for inhalable proteins and peptides.
The dry powder inhalers segment held a 17.1% CAGR in 2024. DPIs enjoy significant market share due to their excellent stability, as biologics can be formulated in a dry state, avoiding the need for cold chain storage required by liquid formulations. This characteristic makes DPIs especially valuable for manufacturers targeting global distribution, including regions with limited refrigeration infrastructure. DPIs also offer a convenient, portable, and user-friendly option compared to nebulizers and MDIs, leading to improved patient adherence, especially for long-term treatment of chronic diseases. The capacity of DPIs to deliver therapeutic biologics deeply into the lungs-such as monoclonal antibodies, vaccines, and peptides-boosts their therapeutic effectiveness and supports their widespread adoption.
U.S. Inhalable Biologics Market accounted for USD 1.4 billion in 2024 and is growing steadily at a CAGR of 16.7%. The increasing pollution levels in the U.S. are contributing to a rise in respiratory problems like asthma, allergies, and nasal congestion. Heightened consumer awareness about air pollution's detrimental effects on respiratory health is driving demand for inhalable biologics as preventive and therapeutic options. The U.S. FDA plays a pivotal role by encouraging the development and approval of inhalable biologics through regulatory frameworks and incentive programs such as the Orphan Drug Designation. This regulatory environment, combined with growing patient demand and innovation, is propelling market growth in the country.
Leading companies competing in the Global Inhalable Biologics Market include AstraZeneca, Mannkind, United Therapeutics, Chiesi Pharmaceuticals, Merxin, Boehringer Ingelheim, Kamada Pharmaceuticals, Teva Pharmaceutical, AbbVie, CanSino Biologics, and F-Hoffman Roche. These key players continue to drive innovation and market expansion globally. To secure and enhance their market positions, companies in the inhalable biologics sector emphasize continuous research and development focused on improving drug delivery technologies and biologic formulation stability. They invest in next-generation inhaler devices that maximize drug deposition efficiency and patient compliance while minimizing side effects. Strategic collaborations and partnerships with regulatory agencies, research institutions, and healthcare providers help accelerate product approvals and expand market reach.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
- 2.2 Key market trends
- 2.2.1 Regional
- 2.2.2 Product type
- 2.2.3 Application
- 2.2.4 Dosage form
- 2.2.5 Distribution channel
- 2.3 CXO perspectives: Strategic imperatives
- 2.3.1 Key decision points for industry executives
- 2.3.2 Critical success factors for market players
- 2.4 Future outlook and strategic recommendations
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.1.1 Supplier landscape
- 3.1.2 Value addition at each stage
- 3.1.3 Factors affecting the value chain
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Rising prevalence of respiratory diseases
- 3.2.1.2 Increasing preference for non-invasive drug delivery methods
- 3.2.1.3 Growing demand for home-based and self-administered therapies
- 3.2.1.4 Technological advancements in inhalation devices and biologics
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 Limited stability and shelf-life of certain inhalable biologics
- 3.2.2.2 High development and production costs
- 3.2.3 Market opportunities
- 3.2.3.1 Growing investment in local manufacturing in emerging markets
- 3.2.3.2 Public-private partnerships for access and affordability
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.4.1 North America
- 3.4.2 Europe
- 3.4.3 Asia Pacific
- 3.4.4 Latin America
- 3.4.5 Middle East and Africa
- 3.5 Pipeline analysis
- 3.6 Future market trends
- 3.7 Technology landscape
- 3.7.1 Current technological trends
- 3.7.2 Emerging technologies
- 3.8 Porter's analysis
- 3.9 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.3 Company matrix analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Key developments
- 4.6.1 Merger and acquisition
- 4.6.2 Partnership and collaboration
- 4.6.3 New product launches
- 4.6.4 Expansion plans
Chapter 5 Market Estimates and Forecast, By Product Type, 2021 – 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Proteins and peptides
- 5.3 Vaccines
- 5.4 Monoclonal antibodies
Chapter 6 Market Estimates and Forecast, By Application, 2021 – 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Respiratory diseases
- 6.3 Infectious diseases
- 6.4 Diabetes
Chapter 7 Market Estimates and Forecast, By Dosage Form, 2021 – 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Dry powder inhalers
- 7.3 Metered dose inhalers
- 7.4 Nebulizers
- 7.5 Soft mist inhalers
Chapter 8 Market Estimates and Forecast, By Distribution Channel, 2021 – 2034 ($ Mn)
- 8.1 Key trends
- 8.2 Hospital pharmacies
- 8.3 Retail pharmacies
- 8.4 Online pharmacies
Chapter 9 Market Estimates and Forecast, By Region, 2021 – 2034 ($ Mn)
- 9.1 Key trends
- 9.2 North America
- 9.2.1 U.S.
- 9.2.2 Canada
- 9.3 Europe
- 9.3.1 Germany
- 9.3.2 UK
- 9.3.3 France
- 9.3.4 Spain
- 9.3.5 Italy
- 9.3.6 Netherlands
- 9.4 Asia Pacific
- 9.4.1 China
- 9.4.2 Japan
- 9.4.3 India
- 9.4.4 Australia
- 9.4.5 South Korea
- 9.5 Latin America
- 9.5.1 Brazil
- 9.5.2 Mexico
- 9.5.3 Argentina
- 9.6 Middle East and Africa
- 9.6.1 South Africa
- 9.6.2 Saudi Arabia
- 9.6.3 UAE
Chapter 10 Company Profiles
- 10.1 AbbVie
- 10.2 AstraZeneca
- 10.3 Boehringer Ingelheim
- 10.4 CanSino Biologics
- 10.5 Chiesi Pharmaceuticals
- 10.6 F-Hoffman Roche
- 10.7 Kamada Pharmaceuticals
- 10.8 Mannkind
- 10.9 Merxin
- 10.10 Teva Pharmaceutical
- 10.11 United Therapeutics









