 |
市场调查报告书
商品编码
1773386
NUT 中线癌治疗市场机会、成长动力、产业趋势分析及 2025 - 2034 年预测NUT Midline Carcinoma Treatment Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
2024年,全球NUT中线癌治疗市场规模达202亿美元,预计到2034年将以10.7%的复合年增长率成长,达到552亿美元。由于NMC在全球的盛行率不断上升,以及诊断和治疗技术的进步,该市场正在迅速扩张。政府扶持政策、临床试验资金以及旨在对抗罕见癌症的措施等关键因素正在推动这一成长。
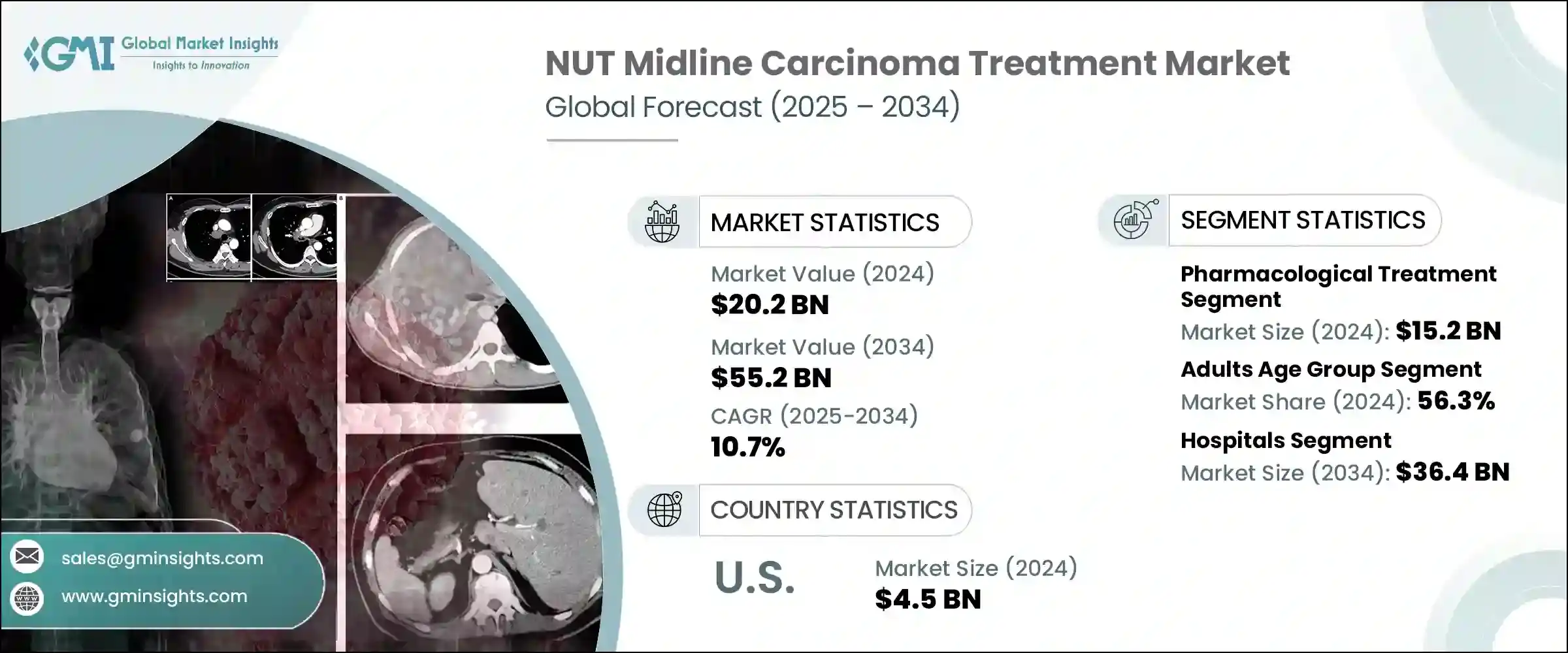
此外,先进诊断工具的普及有助于医疗专业人员更早发现NMC,从而提高治疗率。此外,新兴市场的城市化、医疗保健可近性的改善以及可支配收入的提高也提高了诊断率,进一步推动了市场扩张。政府和非政府来源的研究经费增加,以及对标靶疗法等新型疗法开发的持续关注,也对市场成长发挥重要作用。
| 市场范围 | |
|---|---|
| 起始年份 | 2024 |
| 预测年份 | 2025-2034 |
| 起始值 | 202亿美元 |
| 预测值 | 552亿美元 |
| 复合年增长率 | 10.7% |
专科肿瘤医院和先进诊断中心数量的不断增长,显着促进了NUT中线癌的早期发现和改善管理,增强了市场的整体发展势头。这些机构越来越多地配备了分子影像、精准活检系统和基因定序工具等先进技术,使临床医生能够更准确地识别复杂的基因异常。这种诊断精准度不仅加速了标靶治疗的启动,还改善了预后,这对于像NMC这样的罕见且侵袭性癌症来说是一个关键因素。
2024年,药物治疗领域产值达152亿美元。分子诊断技术的进步,例如新一代定序 (NGS) 和萤光原位杂交 (FISH),使得能够精确识别NUTM1基因重排,从而实现更有针对性的药物治疗。此外,NMC的日益普及、诊断能力的提升以及对改进治疗方案的需求不断增长,正在推动市场成长。该领域也受益于新型疗法的开发,例如BET抑制剂和靶向BRD-NUT融合蛋白的药物,这些药物在早期试验中显示出良好的疗效。
2024年,成人市场占最大份额,达56.3%。虽然NMC最初被认为主要影响儿童和青少年,但最近的研究表明,成年人,尤其是20至50岁人口的发病率更高。这种人口结构的变化,加上医疗保健和分子分析工具的普及,使得成人诊断更加便利、准确。这反过来又提高了治疗的接受度,尤其是BET抑制剂和免疫疗法等先进疗法。
2024年,美国NUT中线癌症治疗市场规模达45亿美元。美国进行了许多针对罕见癌症(包括NMC)的临床试验。领先的机构正引领标靶疗法的开发,例如BET抑制剂和免疫疗法。此外,美国食品药物管理局(FDA)已授予多种NMC疗法孤儿药资格,并提供税收抵免、市场独占权和加速审批途径等激励措施。这些激励措施鼓励製药公司加大对NMC研发的投资,进一步推动市场成长。
NUT 中线癌治疗市场的主要参与者包括默克公司、C4 Therapeutics、Constellation Pharmaceuticals、辉瑞公司、Syndax Pharmaceuticals、百时美施贵宝公司、葛兰素史克公司、罗氏公司、益普生生物製药公司和 OncoFusion Therapeutics。 NMC 治疗市场的公司致力于透过大力投资研发来巩固其市场地位,以开发能够解决 NMC 分子复杂性的标靶疗法。
与学术和研究机构的合作使这些公司能够加快临床试验,而采用先进的诊断技术则有助于他们更深入地了解疾病。此外,各公司正专注于孤儿药地位和快速审批等监管途径,以便更快地将新疗法推向市场。此外,与拥有创新治疗方案的小型生物技术公司建立合作伙伴关係并进行策略性收购,正在帮助大型企业扩大其产品组合,并为NMC(神经胶质母细胞瘤)的治疗方案提供多样化选择。
目录
第一章:方法论与范围
第二章:执行摘要
第三章:行业洞察
- 产业生态系统分析
- 供应商格局
- 每个阶段的增值
- 影响价值链的因素
- 产业衝击力
- 成长动力
- 侦测 NMC 的先进诊断技术
- 医院和专科肿瘤中心不断扩张
- 治疗方式不断进步
- 产业陷阱与挑战
- 治疗费用高昂
- 治疗选择有限
- 市场机会
- 越来越关注标靶治疗
- 增加临床试验的投资
- 成长动力
- 成长潜力分析
- 监管格局
- 北美洲
- 欧洲
- 亚太地区
- 技术格局
- 未来市场趋势
- 管道分析
- 波特的分析
- PESTEL分析
第四章:竞争格局
- 介绍
- 公司矩阵分析
- 公司市占率分析
- 主要市场参与者的竞争分析
- 竞争定位矩阵
- 关键进展
- 併购
- 伙伴关係与协作
- 新产品发布
第五章:市场估计与预测:按治疗类型,2021 - 2034 年
- 主要趋势
- 药物治疗
- 透过治疗
- 化疗
- 免疫疗法
- 其他类型
- 依给药途径
- 口服
- 肠外
- 透过治疗
- 非药物治疗
第六章:市场估计与预测:按年龄组,2021 - 2034 年
- 主要趋势
- 儿科
- 成年人
第七章:市场估计与预测:依最终用途,2021 - 2034 年
- 主要趋势
- 医院
- 专科肿瘤诊所
- 居家照护环境
- 其他最终用途
第八章:市场估计与预测:按地区,2021 - 2034 年
- 主要趋势
- 北美洲
- 我们
- 加拿大
- 欧洲
- 德国
- 英国
- 法国
- 西班牙
- 义大利
- 荷兰
- 亚太地区
- 中国
- 印度
- 日本
- 澳洲
- 韩国
- 拉丁美洲
- 巴西
- 墨西哥
- 阿根廷
- 中东和非洲
- 南非
- 沙乌地阿拉伯
- 阿联酋
第九章:公司简介
- Bristol-Myers Squibb Company
- C4 Therapeutics
- Constellation Pharmaceuticals
- F. Hoffmann-La Roche
- GlaxoSmithKline
- Ipsen Biopharmaceuticals
- Merck & Co
- OncoFusion Therapeutics
- Pfizer
- Syndax Pharmaceuticals
The Global NUT Midline Carcinoma Treatment Market was valued at USD 20.2 billion in 2024 and is estimated to grow at a CAGR of 10.7% to reach USD 55.2 billion by 2034. The market is expanding rapidly due to the rising global prevalence of NMC, coupled with advancements in diagnostic and therapeutic technologies. Key factors such as supportive government policies, funding for clinical trials, and initiatives aimed at combating rare cancers are driving this growth.

Moreover, the increased availability of advanced diagnostic tools is helping healthcare professionals detect NMC earlier, contributing to higher treatment uptake. Additionally, urbanization, improved healthcare access, and higher disposable incomes in emerging markets are enhancing diagnostic rates, further fueling market expansion. Increased research funding from both governmental and non-governmental sources, along with the continuous focus on developing novel treatments like targeted therapies, are also playing significant roles in market growth.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $20.2 Billion |
| Forecast Value | $55.2 Billion |
| CAGR | 10.7% |
The growing number of specialized oncology hospitals and advanced diagnostic centers is significantly contributing to earlier detection and improved management of NUT midline carcinoma, reinforcing the market's overall momentum. These facilities are increasingly equipped with state-of-the-art technologies like molecular imaging, precision biopsy systems, and genetic sequencing tools that allow clinicians to pinpoint complex genetic abnormalities with higher accuracy. This diagnostic precision not only accelerates the initiation of targeted treatments but also enhances prognosis, which is a critical factor in rare and aggressive cancers like NMC.
The pharmacological treatment segment generated USD 15.2 billion in 2024. Technological advancements in molecular diagnostics, such as next-generation sequencing (NGS) and fluorescence in situ hybridization (FISH), have enabled precise identification of NUTM1 gene rearrangements, allowing for more targeted pharmacological treatments. Furthermore, the growing prevalence of NMC, increased diagnostic capabilities, and the rising demand for improved therapeutics options are boosting market growth. The segment is also benefitting from the development of novel therapies such as BET inhibitors and agents targeting BRD-NUT fusion proteins, which are showing promising results in early-stage trials.
In 2024, the adult segment accounted for the largest market share, with 56.3%. While NMC was initially thought to affect primarily children and adolescents, more recent studies have shown a higher incidence among adults, particularly those aged 20 to 50. This demographic shift, combined with better access to healthcare and molecular profiling tools, has led to earlier and more accurate diagnoses in adults. This, in turn, has resulted in higher treatment uptake, particularly for advanced therapies like BET inhibitors and immunotherapies.
U.S. NUT Midline Carcinoma Treatment Market was valued at USD 4.5 billion in 2024. The country is home to numerous clinical trials focused on rare cancers, including NMC. Leading institutions are spearheading the development of targeted therapies like BET inhibitors and immunotherapies. Furthermore, the U.S. FDA has granted orphan drug designations to several NMC treatments, offering incentives like tax credits, market exclusivity, and accelerated approval pathways. These incentives are encouraging pharmaceutical companies to increase their investment in NMC research and development, further propelling market growth.
Key players in the NUT Midline Carcinoma Treatment Market include Merck & Co, C4 Therapeutics, Constellation Pharmaceuticals, Pfizer, Syndax Pharmaceuticals, Bristol-Myers Squibb Company, GlaxoSmithKline, F. Hoffmann-La Roche, Ipsen Biopharmaceuticals, OncoFusion Therapeutics. Companies in the NMC treatment market focus on strengthening their position by investing heavily in research and development to create targeted therapies that address the molecular intricacies of NMC.
Collaborations with academic and research institutions allow these companies to accelerate clinical trials, while the adoption of advanced diagnostic technologies helps them gain a deeper understanding of the disease. Furthermore, companies are focusing on regulatory pathways like orphan drug status and fast-track approval to bring new treatments to market faster. Additionally, partnerships and strategic acquisitions of smaller biotech firms with innovative therapeutic solutions are helping larger players expand their product portfolios and diversify treatment options for NMC.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definition
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 360° synopsis
- 2.2 Key market trends
- 2.2.1 Regional
- 2.2.2 Disease type
- 2.2.3 Treatment type
- 2.2.4 Age group
- 2.2.5 End use
- 2.3 CXO perspectives: Strategic imperatives
- 2.3.1 Key decision points for industry executives
- 2.3.2 Critical success factors for market players
- 2.4 Future outlook and strategic recommendations
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.1.1 Supplier landscape
- 3.1.2 Value addition at each stage
- 3.1.3 Factor affecting the value chain
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Availability of advanced diagnostic techniques to detect NMC
- 3.2.1.2 Increasing expansion of hospitals and specialty oncology centers
- 3.2.1.3 Growing advancement in treatment modalities
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 High cost of treatment
- 3.2.2.2 Limited treatment options
- 3.2.3 Market opportunities
- 3.2.3.1 Growing focus on targeted therapies
- 3.2.3.2 Increasing investment in clinical trials
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.4.1 North America
- 3.4.2 Europe
- 3.4.3 Asia Pacific
- 3.5 Technology landscape
- 3.6 Future market trends
- 3.7 Pipeline analysis
- 3.8 Porter's analysis
- 3.9 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company matrix analysis
- 4.3 Company market share analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Key developments
- 4.6.1 Merger and acquisition
- 4.6.2 Partnership and collaboration
- 4.6.3 New product launches
Chapter 5 Market Estimates and Forecast, By Treatment Type, 2021 - 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Pharmacological treatment
- 5.2.1 By therapy
- 5.2.1.1 Chemotherapy
- 5.2.1.2 Immunotherapy
- 5.2.1.3 Other types
- 5.2.2 By route of administration
- 5.2.2.1 Oral
- 5.2.2.2 Parenteral
- 5.2.1 By therapy
- 5.3 Non-pharmacological treatment
Chapter 6 Market Estimates and Forecast, By Age Group, 2021 - 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Pediatric
- 6.3 Adults
Chapter 7 Market Estimates and Forecast, By End Use, 2021 - 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Hospitals
- 7.3 Specialty oncology clinics
- 7.4 Homecare settings
- 7.5 Other end use
Chapter 8 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Mn)
- 8.1 Key trends
- 8.2 North America
- 8.2.1 U.S.
- 8.2.2 Canada
- 8.3 Europe
- 8.3.1 Germany
- 8.3.2 UK
- 8.3.3 France
- 8.3.4 Spain
- 8.3.5 Italy
- 8.3.6 Netherlands
- 8.4 Asia Pacific
- 8.4.1 China
- 8.4.2 India
- 8.4.3 Japan
- 8.4.4 Australia
- 8.4.5 South Korea
- 8.5 Latin America
- 8.5.1 Brazil
- 8.5.2 Mexico
- 8.5.3 Argentina
- 8.6 Middle East and Africa
- 8.6.1 South Africa
- 8.6.2 Saudi Arabia
- 8.6.3 UAE
Chapter 9 Company Profiles
- 9.1 Bristol-Myers Squibb Company
- 9.2 C4 Therapeutics
- 9.3 Constellation Pharmaceuticals
- 9.4 F. Hoffmann-La Roche
- 9.5 GlaxoSmithKline
- 9.6 Ipsen Biopharmaceuticals
- 9.7 Merck & Co
- 9.8 OncoFusion Therapeutics
- 9.9 Pfizer
- 9.10 Syndax Pharmaceuticals






