 |
市场调查报告书
商品编码
1782150
乙肝疫苗市场机会、成长动力、产业趋势分析及2025-2034年预测Hepatitis B Vaccine Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
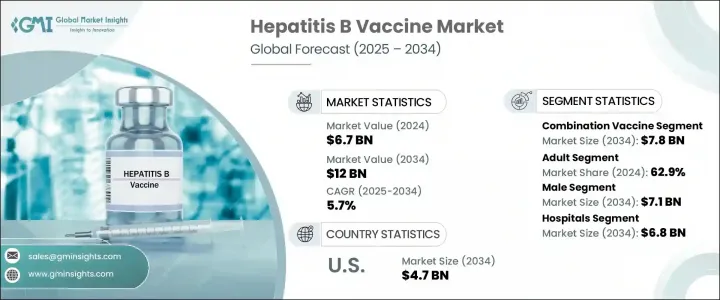
2024 年全球乙肝疫苗市场价值为 67 亿美元,预计到 2034 年将以 5.7% 的复合年增长率成长,达到 120 亿美元。这一成长轨迹很大程度上受到全球乙肝病例不断增加的影响,这得益于政府支持的疫苗接种运动和疫苗研发力度的加速。国家免疫计划和公共卫生意识的提高,使得不同年龄层的人群能够更广泛地获得和接种疫苗。此外,对更有效率、更有针对性的解决方案的需求正在推动疫苗配方和生产的不断创新。重组技术和递送机制的进步有助于提高疫苗效力、延长免疫持续时间并减少剂量需求。随着医疗保健系统致力于减轻肝臟相关疾病的负担,市场对预防性疫苗接种的需求正在增强,尤其是在高风险族群中。
人们对能够提供强大而快速免疫反应的新一代疫苗製剂日益增长的偏好,进一步推动了全球范围内的推广。这些先进的疫苗经过精心设计,具有增强的抗原稳定性、改进的佐剂系统和优化的给药方式,能够更快、更持久地预防B型肝炎病毒。与传统製剂不同,新一代疫苗旨在减少完全免疫所需的剂量,使其更适合大规模免疫接种活动,尤其是在医疗保健资源有限的地区。这项创新对于免疫功能低下者和可能无法有效应答传统疫苗的高危险群尤为重要。
| 市场范围 | |
|---|---|
| 起始年份 | 2024 |
| 预测年份 | 2025-2034 |
| 起始值 | 67亿美元 |
| 预测值 | 120亿美元 |
| 复合年增长率 | 5.7% |
2024年,联合疫苗市场规模达45亿美元,位居市场首位。预计到2034年,该市场规模将达到78亿美元,复合年增长率为5.5%。这些多价疫苗将乙肝疫苗与其他传染源的免疫接种相结合,减少了所需的注射次数。这种整合方法提高了患者的依从性,并简化了疫苗接种程序,尤其适用于婴幼儿。因此,联合疫苗在儿科医疗保健领域得到了广泛应用,并得到了公共和私人免疫计划的支持。由于其高效的接种效率、更广泛的覆盖范围和成本优势,越来越多的政府和医疗机构选择这些疫苗,这有助于提高整个社区的疫苗接种率。
预计到2034年,男性市场规模将达到71亿美元。这主要是由于男性生理和荷尔蒙水平的差异,这些差异增加了慢性感染和肝臟相关併发症的风险。睪固酮已被证明会影响免疫反应和病毒的持久性,使男性长期感染B型肝炎病毒的风险更高。因此,免疫接种计划越来越侧重于成年男性,成人疫苗製剂的广泛普及也进一步推动了这一趋势。意识的增强、医疗保健服务的普及以及对预防保健的推动,将继续加速该领域的疫苗普及。
2024年,北美乙肝疫苗市场规模达27亿美元,预计2034年将达47亿美元,复合年增长率为5.4%。凭藉先进的医疗体系和高度的公众认知度,该地区仍是全球乙肝疫苗领域的主导力量。强大的基础设施以及政府支持的广泛疫苗接种计划,使得儿童和成人的免疫接种率始终保持高位。乙肝的负担仍然很沉重,尤其是在弱势群体中,这促使美国和加拿大等国家持续努力,以扩大预防范围并实现早期免疫覆盖。
主要市场参与者包括印度血清研究所、VBI疫苗公司、赛诺菲、Biological E、YS Biopharma、葛兰素史克、深圳康泰生物製品有限公司、巴拉特生物技术公司、Dynavax Technologies、默克公司和吉利德科学公司。为了巩固市场地位,B型肝炎疫苗领域的公司正专注于产品创新、扩大全球分销网络并建立策略合作伙伴关係。
领先的企业正在大力投入研发,以开发安全性更高、免疫原性更强的下一代疫苗。许多企业正在利用联合疫苗平台来扩大覆盖范围,并吸引更广泛的医疗体系。与政府卫生机构、非政府组织和国际组织的合作也使疫苗在新兴市场的推广更加顺畅。
目录
第一章:方法论与范围
第二章:执行摘要
第三章:行业洞察
- 产业生态系统分析
- 供应商格局
- 每个阶段的增值
- 影响价值链的因素
- 产业衝击力
- 成长动力
- 肝病盛行率上升
- 扩大免疫覆盖计划
- 疫苗配方和生产效率的进步
- 增加研发投入和活动
- 产业陷阱与挑战
- 严格的监管审批流程
- 疫苗储存和运输成本高
- 市场机会
- 加强疫苗分发的公私伙伴关係
- 扩大成人疫苗接种计划
- 成长动力
- 成长潜力分析
- 管道分析
- 监管格局
- 技术进步
- 未来市场趋势
- 波特的分析
- PESTEL分析
第四章:竞争格局
- 介绍
- 公司矩阵分析
- 公司市占率分析
- 主要市场参与者的竞争分析
- 竞争定位矩阵
- 关键进展
- 併购
- 伙伴关係和合作
- 扩张计划
第五章:市场估计与预测:按疫苗类型,2021 - 2034 年
- 主要趋势
- 单抗原疫苗
- 联合疫苗
第六章:市场估计与预测:按年龄组,2021 - 2034 年
- 主要趋势
- 儿科
- 成人
第七章:市场估计与预测:依性别,2021 - 2034 年
- 主要趋势
- 女性
- 男性
第八章:市场估计与预测:依最终用途,2021 - 2034 年
- 主要趋势
- 医院
- 民众
- 私人的
- 专科诊所
- 其他最终用途
第九章:市场估计与预测:按地区,2021 - 2034 年
- 主要趋势
- 北美洲
- 我们
- 加拿大
- 欧洲
- 德国
- 英国
- 法国
- 西班牙
- 义大利
- 荷兰
- 亚太地区
- 中国
- 日本
- 印度
- 澳洲
- 韩国
- 拉丁美洲
- 巴西
- 墨西哥
- 阿根廷
- 中东和非洲
- 南非
- 沙乌地阿拉伯
- 阿联酋
第十章:公司简介
- Bharat Biotech
- Biological E
- Dynavax Technologies
- Gilead Sciences
- GSK
- Merck
- Sanofi
- Serum Institute of India
- Shenzhen Kangtai Biological Products
- VBI vaccines
- YS Biopharma
The Global Hepatitis B Vaccine Market was valued at USD 6.7 billion in 2024 and is estimated to grow at a CAGR of 5.7% to reach USD 12 billion by 2034. The growth trajectory is largely influenced by the increasing number of hepatitis B cases worldwide, backed by government-backed vaccination campaigns and accelerated vaccine development efforts. National immunization programs and expanded public health awareness have led to greater access and uptake of vaccines across various age groups. Additionally, demand for more efficient and targeted solutions is pushing continuous innovation in vaccine formulation and manufacturing. Advances in recombinant technology and delivery mechanisms are contributing to higher efficacy, longer-lasting immunity, and reduced dosage requirements. As healthcare systems aim to reduce the burden of liver-related diseases, the market is witnessing stronger momentum for preventive vaccination, particularly among at-risk populations.

The rising preference for next-generation formulations that deliver robust and rapid immune responses further supports adoption globally. These advanced vaccines are being engineered with enhanced antigen stability, improved adjuvant systems, and optimized delivery methods to generate quicker and more sustained protection against hepatitis B. Unlike traditional formulations, next-gen vaccines aim to reduce the number of doses required for full immunity, making them more suitable for large-scale immunization campaigns, especially in regions with limited healthcare access. This innovation is particularly crucial for immunocompromised individuals and high-risk groups who may not respond effectively to conventional vaccines.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $6.7 Billion |
| Forecast Value | $12 Billion |
| CAGR | 5.7% |
In 2024, the combination vaccines segment led the market with a value of USD 4.5 billion and is forecasted to reach USD 7.8 billion by 2034, growing at a CAGR of 5.5%. These multivalent vaccines combine hepatitis B protection with immunization against other infectious agents, reducing the number of injections required. This integrated approach improves patient compliance and simplifies vaccine schedules, especially for infants and children. As a result, combination vaccines have gained widespread adoption in pediatric healthcare, supported by both public and private immunization programs. Governments and providers increasingly opt for these vaccines due to their efficiency in administration, broader coverage, and cost advantages, helping raise vaccination rates across communities.
The male segment is expected to reach USD 7.1 billion by 2034. This is largely driven by biological and hormonal differences that increase the risk of chronic infection and liver-related complications in men. Testosterone has been shown to influence immune response and virus persistence, placing males at a greater risk of prolonged hepatitis B infection. As a result, immunization programs have increasingly focused on adult men, with widespread availability of adult vaccine formulations supporting this trend. Growing awareness, better access to healthcare, and a push for preventive care continue to accelerate vaccine adoption in this segment.
North America Hepatitis B Vaccine Market was valued at USD 2.7 billion in 2024 and is projected to reach USD 4.7 billion by 2034 at a CAGR of 5.4% through 2034. The region remains a dominant force in the global hepatitis B vaccine landscape due to its advanced healthcare delivery systems and high public awareness. Strong infrastructure, along with extensive government-backed vaccination programs, has enabled both pediatric and adult immunization rates to remain consistently high. The burden of hepatitis B remains significant, especially in vulnerable populations, prompting sustained efforts toward widespread prevention and early immunization coverage in countries like the U.S. and Canada.
Key market players include Serum Institute of India, VBI Vaccines, Sanofi, Biological E, YS Biopharma, GSK, Shenzhen Kangtai Biological Products, Bharat Biotech, Dynavax Technologies, Merck, and Gilead Sciences. To strengthen their market position, companies in the hepatitis B vaccine segment are focusing on product innovation, expanding their global distribution networks, and engaging in strategic partnerships.
Leading players are investing heavily in R&D to develop next-generation vaccines with improved safety profiles and stronger immunogenicity. Many are leveraging combination vaccine platforms to increase coverage and appeal to broader healthcare systems. Collaborations with government health agencies, NGOs, and international organizations are also enabling smoother vaccine rollouts in emerging markets.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definition
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/Country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
- 2.2 Key market trends
- 2.2.1 Regional
- 2.2.2 Vaccine type
- 2.2.3 Age group
- 2.2.4 Gender
- 2.2.5 End use
- 2.3 CXO perspectives: Strategic imperatives
- 2.3.1 Key decision points for industry executives
- 2.3.2 Critical success factors for market players
- 2.4 Future outlook and strategic recommendations
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.1.1 Supplier landscape
- 3.1.2 Value addition at each stage
- 3.1.3 Factors affecting the value chain
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Rising prevalence of liver diseases
- 3.2.1.2 Growing immunization coverage programs
- 3.2.1.3 Advancements in vaccine formulation and production efficiency
- 3.2.1.4 Increasing R&D investment and activities
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 Stringent regulatory approval processes
- 3.2.2.2 High cost of storage and transportation of vaccine
- 3.2.3 Market opportunities
- 3.2.3.1 Growing public-private partnerships for vaccine distribution
- 3.2.3.2 Expanding adult vaccination programs
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Pipeline analysis
- 3.5 Regulatory landscape
- 3.6 Technological advancements
- 3.7 Future market trends
- 3.8 Porter’s analysis
- 3.9 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company matrix analysis
- 4.3 Company market share analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Key developments
- 4.6.1 Mergers and acquisitions
- 4.6.2 Partnerships and collaborations
- 4.6.3 Expansion plans
Chapter 5 Market Estimates and Forecast, By Vaccine Type, 2021 - 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Single antigen vaccine
- 5.3 Combination vaccine
Chapter 6 Market Estimates and Forecast, By Age Group, 2021 - 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Pediatric
- 6.3 Adult
Chapter 7 Market Estimates and Forecast, By Gender, 2021 - 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Female
- 7.3 Male
Chapter 8 Market Estimates and Forecast, By End Use, 2021 - 2034 ($ Mn)
- 8.1 Key trends
- 8.2 Hospitals
- 8.2.1 Public
- 8.2.2 Private
- 8.3 Specialty clinics
- 8.4 Other end use
Chapter 9 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Mn)
- 9.1 Key trends
- 9.2 North America
- 9.2.1 U.S.
- 9.2.2 Canada
- 9.3 Europe
- 9.3.1 Germany
- 9.3.2 UK
- 9.3.3 France
- 9.3.4 Spain
- 9.3.5 Italy
- 9.3.6 Netherlands
- 9.4 Asia Pacific
- 9.4.1 China
- 9.4.2 Japan
- 9.4.3 India
- 9.4.4 Australia
- 9.4.5 South Korea
- 9.5 Latin America
- 9.5.1 Brazil
- 9.5.2 Mexico
- 9.5.3 Argentina
- 9.6 Middle East and Africa
- 9.6.1 South Africa
- 9.6.2 Saudi Arabia
- 9.6.3 UAE
Chapter 10 Company Profiles
- 10.1 Bharat Biotech
- 10.2 Biological E
- 10.3 Dynavax Technologies
- 10.4 Gilead Sciences
- 10.5 GSK
- 10.6 Merck
- 10.7 Sanofi
- 10.8 Serum Institute of India
- 10.9 Shenzhen Kangtai Biological Products
- 10.10 VBI vaccines
- 10.11 YS Biopharma








