 |
市场调查报告书
商品编码
1797821
缝合锚装置市场机会、成长动力、产业趋势分析及 2025 - 2034 年预测Suture Anchor Devices Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
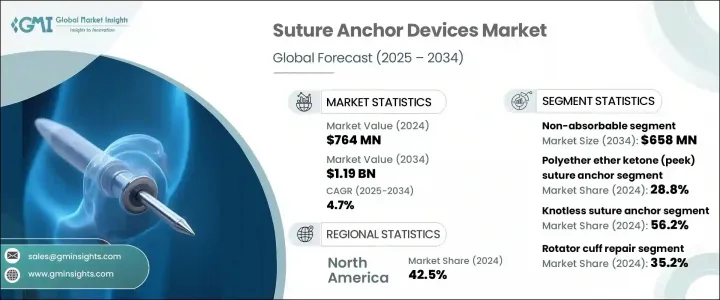
2024 年全球缝合锚装置市场价值为 7.64 亿美元,预计到 2034 年将以 4.7% 的复合年增长率增长至 11.9 亿美元。这一增长主要源于运动伤害发生率的上升,以及肩袖修復、跟腱病治疗和十字韧带重建等手术的增多,这些手术通常都使用缝合锚装置。这些植入物在骨科手术中起着至关重要的作用,它们将肌腱和韧带等软组织连接到骨骼上。老年人口的不断增长也对市场成长做出了重大贡献,因为与年龄相关的疾病需要更多的手术和介入。该行业的领导者包括 Arthrex、Zimmer Biomet、Smith & Nephew 和 Stryker。随着手术技术和材料的创新,缝合锚装置不断发展,以提高固定强度、生物相容性和易用性。
缝合锚是现代骨科和运动医学中必不可少的工具,用于在手术过程中将软组织固定到骨骼上。材料科学的进步促进了机械强度和生物相容性更高的缝合锚的开发,从而确保了更好的疗效和更高的患者满意度。这些装置是微创手术不可或缺的一部分,在微创手术中,精准度和组织保存对于术后成功恢復至关重要。随着人们越来越重视缩短恢復时间和提高手术精准度,对高品质缝合锚装置的需求也日益增长。
| 市场范围 | |
|---|---|
| 起始年份 | 2024 |
| 预测年份 | 2025-2034 |
| 起始值 | 7.64亿美元 |
| 预测值 | 11.9亿美元 |
| 复合年增长率 | 4.7% |
2024年,不可吸收缝合锚固装置市场占据主导地位,价值4.261亿美元。预计到2034年,该市场规模将达到6.58亿美元,复合年增长率为4.5%。不可吸收缝合锚固装置因其卓越的机械强度和长期固定能力,在肩袖和盂唇修復等高负荷骨科手术中备受青睐。钛和PEEK(聚醚醚酮)等材料具有高生物相容性、射线可透性和稳定的性能,其持续使用正在推动对不可吸收缝合锚固装置的需求。这些材料确保缝合锚定装置长期可靠地工作,为修復的组织提供永久支撑。
PEEK 缝合锚占据最大市场份额,2024 年占 28.8%。 PEEK 锚的广泛应用归功于其卓越的临床表现、安全性以及外科医生对其非金属特性的青睐。 PEEK 锚具有出色的强度和射线可透性,使其成为肩部、膝盖和髋部手术的理想选择。长期研究表明,PEEK 无结锚的性能与可生物降解锚相当,进一步巩固了其作为骨科手术安全可靠选择的地位。
2024年,美国缝合锚装置市场规模达2.998亿美元。美国在先进骨科技术的应用方面处于领先地位,包括机器人辅助手术和基于PEEK(聚醚醚酮)的缝合锚。美国强大的监管框架、高度的公众认知度以及在研发方面的大量投入是市场成长的主要推动力。随着运动伤害和与年龄相关的肌肉骨骼疾病的发病率不断上升,预计美国市场将在公共卫生措施和私营部门技术创新的推动下持续增长。
缝合锚装置市场的主要参与者包括施乐辉 (Smith & Nephew)、史赛克 (Stryker Corporation)、捷迈邦美 (Zimmer Biomet)、Arthrex 和康美 (ConMed)。缝合锚装置市场的公司采用一系列策略来巩固其地位并增加市场份额。一项关键策略是不断创新材料和装置设计,尤其专注于提高用于复杂手术的缝合锚的性能和耐用性。许多公司也透过推出基于 PEEK 和生物可吸收的缝合锚来扩展其产品组合,以满足对这些材料日益增长的需求。另一项策略是与医院、骨科诊所和研究机构建立策略伙伴关係,以确保其产品得到更广泛的应用。此外,透过投资本地製造工厂和分销网络来增强其在新兴市场的影响力是许多领先企业的重点关注点。这些公司还利用机器人辅助手术等技术进步,将缝合锚装置整合到下一代外科手术中,以确保更高的准确性和更快的恢復时间。
目录
第一章:方法论与范围
第二章:执行摘要
第三章:行业洞察
- 产业生态系统分析
- 产业衝击力
- 成长动力
- 运动事故数量上升
- 老年人口不断增加
- 微创手术的需求
- 锚设计的技术进步
- 产业陷阱与挑战
- 高级锚和手术费用高昂
- 术后併发症的风险
- 市场机会
- 人工智慧与机器人辅助手术的融合
- 生物相容性和生物可吸收材料的创新
- 成长动力
- 成长潜力分析
- 监管格局
- 技术进步
- 当前的技术趋势
- 新兴技术
- 供应链分析
- 2024年定价分析
- 未来市场趋势
- 差距分析
- 波特的分析
- PESTEL分析
第四章:竞争格局
- 介绍
- 公司市占率分析
- 公司矩阵分析
- 主要市场参与者的竞争分析
- 竞争定位矩阵
- 关键进展
- 併购
- 伙伴关係和合作
- 新产品发布
- 扩张计划
第五章:市场估计与预测:按产品,2021 - 2034 年
- 主要趋势
- 可吸收
- 不可吸收
第六章:市场估计与预测:按材料,2021 - 2034 年
- 主要趋势
- 金属缝合锚
- 生物可吸收缝合锚
- 聚醚醚酮(PEEK)缝合锚
- 生物复合材料缝合锚
- 全软缝合锚
第七章:市场估计与预测:按捆绑类型,2021 - 2034
- 主要趋势
- 无结缝合锚
- 打结缝合锚
第八章:市场估计与预测:按应用,2021 - 2034 年
- 主要趋势
- 肩袖修復
- 跟腱病修復
- 十字韧带修復
- 肱二头肌肌腱固定术
- 其他应用
第九章:市场估计与预测:依最终用途,2021 - 2034 年
- 主要趋势
- 医院和诊所
- 门诊手术中心
- 其他最终用途
第十章:市场估计与预测:按地区,2021 - 2034 年
- 主要趋势
- 北美洲
- 我们
- 加拿大
- 欧洲
- 德国
- 英国
- 法国
- 西班牙
- 义大利
- 荷兰
- 亚太地区
- 中国
- 印度
- 日本
- 澳洲
- 韩国
- 拉丁美洲
- 巴西
- 墨西哥
- 阿根廷
- 中东和非洲
- 沙乌地阿拉伯
- 南非
- 阿联酋
第 11 章:公司简介
- Anika Therapeutics
- Arthrex
- ConMed
- Enovis Corporation
- Fuse Medical
- Johnson & Johnson
- MJ Surgical
- NeoSys
- Orthomed
- Ossio
- Parcus Medical
- SBM
- Smith & Nephew
- Stryker Corporation
- Teknimed
- Tulpar Medical Solutions
- Zimmer Biomet
The Global Suture Anchor Devices Market was valued at USD 764 million in 2024 and is estimated to grow at a CAGR of 4.7% to reach USD 1.19 billion by 2034. This growth is primarily driven by the increasing incidence of sports-related injuries, as well as the rise in procedures like rotator cuff repair, Achilles tendinosis treatment, and cruciate ligament reconstruction, all of which commonly employ suture anchor devices. These implants play a crucial role in orthopedic surgeries by attaching soft tissues such as tendons and ligaments to bone. The expanding geriatric population is also contributing significantly to market growth, as age-related conditions require more surgeries and interventions. Leading players in the industry include Arthrex, Zimmer Biomet, Smith & Nephew, and Stryker. With innovations in surgical techniques and materials, suture anchor devices continue to evolve to offer improved fixation strength, biocompatibility, and ease of use.

Suture anchors are essential tools in modern orthopedic and sports medicine, designed to fasten soft tissue to bone during surgical procedures. Advancements in materials science have led to the development of anchors with enhanced mechanical strength and biocompatibility, ensuring better outcomes and increased patient satisfaction. These devices are integral to minimally invasive surgeries, where precision and tissue preservation are critical for successful recovery. With a growing emphasis on reducing recovery times and improving surgical precision, the demand for high-quality suture anchor devices continues to rise.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $764 Million |
| Forecast Value | $1.19 Billion |
| CAGR | 4.7% |
The non-absorbable segment dominated the suture anchor devices market in 2024, accounting for USD 426.1 million. This segment is projected to reach USD 658 million by 2034, growing at a CAGR of 4.5%. Non-absorbable anchors are preferred in high-load orthopedic procedures, such as rotator cuff and labral repairs, due to their superior mechanical strength and long-term fixation capabilities. The continued use of materials like titanium and PEEK (polyether ether ketone), which offer high biocompatibility, radiolucency, and consistent performance, is driving the demand for non-absorbable anchors. These materials ensure that the anchors perform reliably over time, providing permanent support to repaired tissues.
The PEEK suture anchor segment held the largest share of the market, accounting for 28.8% in 2024. The widespread adoption of PEEK anchors is attributed to their strong clinical performance, safety profile, and preference among surgeons for their non-metallic nature. PEEK anchors offer excellent strength and radiolucency, making them ideal for use in shoulder, knee, and hip surgeries. Long-term studies have shown that PEEK knotless anchors perform comparably to biodegradable anchors, further cementing their position as a safe and reliable choice for orthopedic procedures.
United States Suture Anchor Devices Market was valued at USD 299.8 million in 2024. The U.S. is at the forefront of adopting advanced orthopedic technologies, including robotic-assisted surgeries and PEEK-based suture anchors. The country's strong regulatory framework, high levels of public awareness, and substantial investments in research and development are major contributors to market growth. With the increasing prevalence of sports injuries and age-related musculoskeletal conditions, the U.S. market is expected to experience sustained growth, driven by both public health initiatives and innovations in private sector technologies.
Major players in the Suture Anchor Devices Market include Smith & Nephew, Stryker Corporation, Zimmer Biomet, Arthrex, and ConMed. Companies in the suture anchor devices market employ a range of strategies to solidify their position and increase market share. A key strategy is the continuous innovation in materials and device design, particularly focusing on improving the performance and durability of suture anchors used in complex surgeries. Many companies are also expanding their product portfolios by introducing PEEK-based and bioabsorbable suture anchors, catering to the growing demand for these materials. Another strategy involves forming strategic partnerships with hospitals, orthopedic clinics, and research institutions to ensure better adoption of their products. In addition, enhancing their presence in emerging markets by investing in local manufacturing facilities and distribution networks is a key focus for many leading players. These companies are also leveraging technological advancements such as robotic-assisted surgery to integrate suture anchor devices into next-gen surgical procedures, ensuring improved accuracy and faster recovery times.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definition
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/Country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
- 2.2 Key market trends
- 2.2.1 Regional trends
- 2.2.2 Product trends
- 2.2.3 Material trends
- 2.2.4 Tying type trends
- 2.2.5 Application trends
- 2.2.6 End use trends
- 2.3 CXO perspectives: Strategic imperatives
- 2.3.1 Key decision points for industry executives
- 2.3.2 Critical success factors for market players
- 2.4 Future outlook and strategic recommendations
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Rising number of sports accidents
- 3.2.1.2 Increasing geriatric population
- 3.2.1.3 Demand for minimally invasive surgeries
- 3.2.1.4 Technological advancements in anchor design
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 High cost of advanced anchors and surgery
- 3.2.2.2 Risk of post operative complications
- 3.2.3 Market opportunities
- 3.2.3.1 Integration of AI and robotic assisted surgery
- 3.2.3.2 Innovation in biocompatible and bioabsorbable materials
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.5 Technological advancements
- 3.5.1 Current technological trends
- 3.5.2 Emerging technologies
- 3.6 Supply chain analysis
- 3.7 Pricing analysis, 2024
- 3.8 Future market trends
- 3.9 Gap analysis
- 3.10 Porter's analysis
- 3.11 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.3 Company matrix analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Key developments
- 4.6.1 Mergers and acquisitions
- 4.6.2 Partnerships and collaborations
- 4.6.3 New product launches
- 4.6.4 Expansion plans
Chapter 5 Market Estimates and Forecast, By Product, 2021 - 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Absorbable
- 5.3 Non absorbable
Chapter 6 Market Estimates and Forecast, By Material, 2021 - 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Metallic suture anchor
- 6.3 Bio absorbable suture anchor
- 6.4 Polyether ether ketone (PEEK) suture anchor
- 6.5 Bio composite suture anchor
- 6.6 All soft suture anchor
Chapter 7 Market Estimates and Forecast, By Tying Type, 2021 - 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Knotless suture anchor
- 7.3 Knotted suture anchor
Chapter 8 Market Estimates and Forecast, By Application, 2021 - 2034 ($ Mn)
- 8.1 Key trends
- 8.2 Rotator cuff repair
- 8.3 Archilles tendinosis repair
- 8.4 Cruciate ligament repairs
- 8.5 Biceps tenodesis
- 8.6 Other applications
Chapter 9 Market Estimates and Forecast, By End Use, 2021 - 2034 ($ Mn)
- 9.1 Key trends
- 9.2 Hospital and clinics
- 9.3 Ambulatory surgical centres
- 9.4 Other end use
Chapter 10 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Mn)
- 10.1 Key trends
- 10.2 North America
- 10.2.1 U.S.
- 10.2.2 Canada
- 10.3 Europe
- 10.3.1 Germany
- 10.3.2 UK
- 10.3.3 France
- 10.3.4 Spain
- 10.3.5 Italy
- 10.3.6 Netherlands
- 10.4 Asia Pacific
- 10.4.1 China
- 10.4.2 India
- 10.4.3 Japan
- 10.4.4 Australia
- 10.4.5 South Korea
- 10.5 Latin America
- 10.5.1 Brazil
- 10.5.2 Mexico
- 10.5.3 Argentina
- 10.6 Middle East and Africa
- 10.6.1 Saudi Arabia
- 10.6.2 South Africa
- 10.6.3 UAE
Chapter 11 Company Profiles
- 11.1 Anika Therapeutics
- 11.2 Arthrex
- 11.3 ConMed
- 11.4 Enovis Corporation
- 11.5 Fuse Medical
- 11.6 Johnson & Johnson
- 11.7 MJ Surgical
- 11.8 NeoSys
- 11.9 Orthomed
- 11.10 Ossio
- 11.11 Parcus Medical
- 11.12 SBM
- 11.13 Smith & Nephew
- 11.14 Stryker Corporation
- 11.15 Teknimed
- 11.16 Tulpar Medical Solutions
- 11.17 Zimmer Biomet








