 |
市场调查报告书
商品编码
1858971
基因治疗市场机会、成长驱动因素、产业趋势分析及预测(2024-2032年)Gene Therapy Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2024 - 2032 |
||||||
2024 年全球基因治疗市场价值为 90 亿美元,预计到 2034 年将以 19.4% 的复合年增长率增长至 513 亿美元。
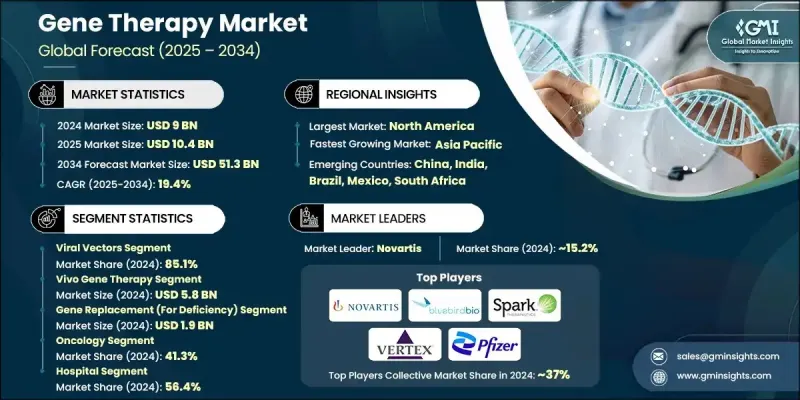
基因递送系统的进步、基因治疗研发投资的增加、基因治疗靶向疾病(如癌症和罕见遗传疾病)的日益普遍以及监管产品审批数量的不断增长,共同推动了基因治疗市场的显着增长。基因和细胞疗法合约生产的普及以及递送技术的策略性发展,进一步提升了市场前景。监管机构正在简化有前景的基因疗法的审批流程,各国政府也在加强对创新基因药物平台的投入。随着医疗保健系统日益关注个人化和标靶治疗,基因疗法因其在分子层面解决复杂疾病的潜力而备受关注。市场参与者也在利用数位化平台共享资料、加速临床试验并优化药物研发流程。同时,精准医疗和生物资讯学的更广泛融合,正帮助基因疗法提高疗效、减少副作用并带来长期的治疗益处。这些趋势正将基因疗法推向现代医学下一阶段的颠覆性发展阶段。
| 市场范围 | |
|---|---|
| 起始年份 | 2024 |
| 预测年份 | 2025-2034 |
| 起始值 | 90亿美元 |
| 预测值 | 513亿美元 |
| 复合年增长率 | 19.4% |
2024年,病毒载体市占率高达85.1%,凸显了其作为最有效递送载体的持续主导地位。这些载体,包括腺相关病毒和慢病毒,因其能够精准递送遗传物质并长期维持稳定的基因表现而被广泛应用。其广泛的临床应用以及在已获批准疗法中取得的成功,进一步巩固了其商业可行性,使其成为基因疗法研发的基石。稳健的生产流程和可扩展的生产能力也为其在各种治疗产品线中的广泛应用提供了支持。
由于其能够系统性地治疗复杂的多器官疾病,体内基因治疗市场预计在2024年将达到58亿美元。体内疗法能够将基因直接导入体内,无需进行细胞萃取和回输。这种方法有助于扩大治疗范围,并降低手术复杂性。非病毒和混合递送系统(例如奈米颗粒和脂质製剂)的进步正在进一步提高标靶精准度和治疗效果。
预计到2024年,北美基因治疗市占率将达到51.2%,成为基因治疗创新和商业化的主导地区。这一领先地位得益于稳健的投资环境、有利的监管框架和完善的医疗基础设施。凭藉领先的生物技术公司、杰出的学术机构和高水准的研发活动,该地区,尤其是美国,正不断拓展基因治疗科学的边界。强大的公私合作和丰富的人才储备正在加速前沿疗法的临床开发和商业化应用。
全球基因治疗市场的主要参与者包括Sangamo Therapeutics、安进(Amgen)、Spark Therapeutics(罗氏旗下)、Beam Therapeutics、Krystal Biotech、Sarepta Therapeutics、AGTC、Orchard Therapeutics、bluebird bio、百时美施贵宝(Bristol-Myers Sbbqui Company(Gile) Sciences)、诺华(Novartis)、Helixmith、辉瑞(Pfizer)、Intellia Therapeutics、拜耳(Bayer)、UniQure NV、BioMarin Pharmaceutical、CRISPR Therapeutics和Audentes Therapeutics(安斯泰来製药旗下)。为了巩固市场地位,基因治疗领域的领导者正优先考虑策略性併购,以增强其技术平台并扩大产品线。许多公司正与学术机构和小型生物技术创新者建立研发合作关係,以获取新型疗法并加速临床试验进程。增加对内部生产能力和全球供应链基础设施的投资,可确保更快的规模生产和合规性。各公司也正加强争取快速通道、孤儿药和突破性疗法认定,以缩短产品上市时间。
目录
第一章:方法论与范围
第二章:执行概要
第三章:行业洞察
- 产业生态系分析
- 产业影响因素
- 成长驱动因素
- 基因编辑技术的进步
- 罕见遗传性疾病和先天性疾病的盛行率不断上升
- 投资和合作不断增加
- 人们对基因诊断的认识不断提高,基因诊断技术也日益进步。
- 产业陷阱与挑战
- 高昂的研发和製造成本
- 基因治疗递送的复杂性与潜在副作用
- 市场机会
- 开发个人化和精准基因疗法
- 随着医疗基础设施的不断发展,新兴市场将迎来扩张。
- 成长驱动因素
- 成长潜力分析
- 监管环境
- 北美洲
- 我们
- 加拿大
- 欧洲
- 亚太地区
- 拉丁美洲
- 中东和非洲
- 北美洲
- 技术格局
- 当前趋势
- 新兴技术
- 管道分析
- 未来市场趋势
- 波特的分析
- PESTEL 分析
第四章:竞争格局
- 介绍
- 公司市占率分析
- 全球的
- 北美洲
- 欧洲
- 亚太地区
- 公司矩阵分析
- 主要市场参与者的竞争分析
- 竞争定位矩阵
- 关键进展
- 併购
- 伙伴关係与合作
- 新产品发布
- 扩张计划
第五章:市场估算与预测:基于Vector的数据,2021-2034年
- 主要趋势
- 病毒载体
- 逆转录病毒载体
- 腺相关病毒载体
- 慢病毒载体
- 其他病毒载体
- 非病毒载体
- 质粒DNA
- 脂质奈米颗粒(LNPs)
- 寡核苷酸
- 其他非病毒载体
第六章:市场估算与预测:以交付方式划分,2021-2034年
- 主要趋势
- 体内基因治疗
- 体外基因治疗
第七章:市场估计与预测:依基因类型划分,2021-2034年
- 主要趋势
- 抗原编码基因
- 细胞激素编码基因
- 肿瘤抑制基因
- 自杀基因
- 基因替代(针对缺陷)
- 生长因子基因
- 受体编码基因
- 其他基因类型
第八章:市场估算与预测:依指示剂划分,2021-2034年
- 主要趋势
- 肿瘤学
- 急性淋巴母细胞白血病(ALL)
- 大B细胞淋巴瘤
- 头颈部鳞状细胞癌
- 黑色素瘤
- 神经系统疾病
- 杜氏肌肉营养不良症(DMD)
- 脊髓性肌肉萎缩症(SMA)
- 血友病A和B
- 肝病
- 遗传性视网膜疾病
- 週边动脉疾病
- 其他迹象
第九章:市场估算与预测:依最终用途划分,2021-2034年
- 主要趋势
- 医院
- 专科诊所和基因治疗中心
- 学术和研究机构
- 其他用途
第十章:市场估计与预测:依地区划分,2021-2034年
- 主要趋势
- 北美洲
- 我们
- 加拿大
- 欧洲
- 德国
- 英国
- 法国
- 西班牙
- 义大利
- 荷兰
- 亚太地区
- 中国
- 印度
- 日本
- 澳洲
- 韩国
- 拉丁美洲
- 巴西
- 墨西哥
- 阿根廷
- 中东和非洲
- 南非
- 沙乌地阿拉伯
- 阿联酋
第十一章:公司简介
- Amgen
- Applied Genetic Technologies Corporation (AGTC)
- Audentes Therapeutics, Inc. (Astellas Pharma)
- Bayer
- Beam Therapeutics
- BioMarin Pharmaceutical
- bluebird bio
- Bristol-Myers Squibb Company
- CRISPR Therapeutics
- Gilead Sciences
- Helixmith
- Intellia Therapeutics
- Krystal Biotech
- Novartis
- Orchard Therapeutics
- Pfizer
- Sangamo Therapeutics
- Sarepta Therapeutics
- Spark Therapeutics (Roche)
- UniQure NV
The Global Gene Therapy Market was valued at USD 9 billion in 2024 and is estimated to grow at a CAGR of 19.4% to reach USD 51.3 billion by 2034.

This significant growth trajectory is being fueled by advances in gene delivery systems, rising investments in gene therapy development, the growing prevalence of diseases targeted by gene therapy, such as cancer and rare genetic disorders, and an increasing number of regulatory product approvals. Expanding access to contract manufacturing for gene and cell therapies, along with strategic developments in delivery technology, are further enhancing the market outlook. Regulatory agencies are streamlining approval processes for promising gene therapies, and governments are boosting funding toward innovative genetic medicine platforms. As healthcare systems focus on personalized and targeted treatments, gene therapy is gaining traction for its potential to address complex diseases at the molecular level. Market players are also leveraging digital platforms to share data, accelerate clinical trials, and optimize drug discovery pipelines. Meanwhile, broader integration of precision medicine and bioinformatics is helping gene therapies achieve better treatment efficacy, reduced side effects, and long-term therapeutic benefits. These trends are positioning gene therapy as a disruptive force in the next phase of modern medicine.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $9 Billion |
| Forecast Value | $51.3 Billion |
| CAGR | 19.4% |
In 2024, the viral vectors segment held an 85.1% share, underscoring their continued dominance as the most effective delivery vehicles. These vectors, including adeno-associated viruses and lentiviruses, are widely used due to their superior ability to deliver genetic material accurately and maintain consistent gene expression over time. Their broad clinical application and proven success in approved treatments have reinforced their commercial viability, making them a cornerstone of gene therapy development. Robust manufacturing protocols and scalable production also support their widespread deployment across therapeutic pipelines.
The in vivo gene therapy segment accounted for USD 5.8 billion in 2024, owing to its systemic approach to treating complex and multi-organ diseases. In vivo methods enable direct gene delivery into the body, eliminating the need for cell extraction and reinfusion. This method supports broader therapeutic access and reduces procedural complexity. Advances in non-viral and hybrid delivery systems-such as nanoparticles and lipid-based formulations are further enhancing targeting precision and treatment outcomes.
North America Gene Therapy Market held a 51.2% share in 2024, making it the dominant region for gene therapy innovation and commercialization. This leadership is supported by a robust investment environment, favorable regulatory frameworks, and a sophisticated healthcare infrastructure. With leading biotech firms, prominent academic institutions, and high R&D activity, the region, particularly the U.S., continues to push the boundaries of gene therapy science. Strong public-private collaborations and a deep talent pool are accelerating clinical development and commercial adoption of cutting-edge therapies.
Prominent players in the Global Gene Therapy Market include Sangamo Therapeutics, Amgen, Spark Therapeutics (Roche), Beam Therapeutics, Krystal Biotech, Sarepta Therapeutics, AGTC, Orchard Therapeutics, bluebird bio, Bristol-Myers Squibb Company, Gilead Sciences, Novartis, Helixmith, Pfizer, Intellia Therapeutics, Bayer, UniQure N.V., BioMarin Pharmaceutical, CRISPR Therapeutics, and Audentes Therapeutics (Astellas Pharma). To strengthen their foothold, leading companies in the gene therapy sector are prioritizing strategic mergers and acquisitions to enhance their technology platforms and expand product pipelines. Many firms are entering collaborative R&D partnerships with academic institutions and smaller biotech innovators to access novel therapies and accelerate clinical timelines. Increasing investment in in-house manufacturing capabilities and global supply chain infrastructure ensures faster scaling and regulatory compliance. Companies are also intensifying their focus on securing fast-track, orphan drug, and breakthrough therapy designations to reduce time-to-market.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definition
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/Country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 360° synopsis
- 2.2 Key market trends
- 2.2.1 Regional trends
- 2.2.2 Vector trends
- 2.2.3 Delivery method trends
- 2.2.4 Gene type trends
- 2.2.5 Indication trends
- 2.2.6 End Use trends
- 2.3 CXO perspectives: Strategic imperatives
- 2.3.1 Key decision points for industry executives
- 2.3.2 Critical success factors for market players
- 2.4 Future outlook and strategic recommendations
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Advancements in gene editing technologies
- 3.2.1.2 Increasing prevalence of rare genetic and inherited diseases
- 3.2.1.3 Rising investments and collaborations
- 3.2.1.4 Growing awareness and improved genetic diagnostics
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 High development and manufacturing costs
- 3.2.2.2 Complexity of gene therapy delivery and potential side effects
- 3.2.3 Market opportunities
- 3.2.3.1 Development of personalized and precision gene therapies
- 3.2.3.2 Expansion in emerging markets with growing healthcare infrastructure
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.4.1 North America
- 3.4.1.1 U.S.
- 3.4.1.2 Canada
- 3.4.2 Europe
- 3.4.3 Asia Pacific
- 3.4.4 Latin America
- 3.4.5 Middle East and Africa
- 3.4.1 North America
- 3.5 Technology landscape
- 3.5.1 Current trends
- 3.5.2 Emerging technologies
- 3.6 Pipeline analysis
- 3.7 Future market trends
- 3.8 Porter's analysis
- 3.9 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.2.1 Global
- 4.2.2 North America
- 4.2.3 Europe
- 4.2.4 Asia Pacific
- 4.3 Company matrix analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Key developments
- 4.6.1 Merger and acquisition
- 4.6.2 Partnership and collaboration
- 4.6.3 New product launches
- 4.6.4 Expansion plans
Chapter 5 Market Estimates and Forecast, By Vector, 2021 - 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Viral vectors
- 5.2.1 Retro viral vectors
- 5.2.2 Adeno-associated virus vectors
- 5.2.3 Lentiviral vectors
- 5.2.4 Other viral vectors
- 5.3 Non-viral vectors
- 5.3.1 Plasmid DNA
- 5.3.2 Lipid nanoparticles (LNPs)
- 5.3.3 Oligonucleotides
- 5.3.4 Other non-viral vectors
Chapter 6 Market Estimates and Forecast, By Delivery Method, 2021 - 2034 ($ Mn)
- 6.1 Key trends
- 6.2 In vivo gene therapy
- 6.3 Ex vivo gene therapy
Chapter 7 Market Estimates and Forecast, By Gene Type, 2021 - 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Antigen-encoding genes
- 7.3 Cytokine-encoding genes
- 7.4 Tumor suppressor genes
- 7.5 Suicide genes
- 7.6 Gene replacement (for deficiency)
- 7.7 Growth factor genes
- 7.8 Receptor-encoding genes
- 7.9 Other gene types
Chapter 8 Market Estimates and Forecast, By Indication, 2021 - 2034 ($ Mn)
- 8.1 Key trends
- 8.2 Oncology
- 8.2.1 Acute Lymphoblastic Leukemia (ALL)
- 8.2.2 Large B-cell lymphoma
- 8.2.3 Head & neck squamous cell carcinoma
- 8.2.4 Melanoma
- 8.3 Neurological disorders
- 8.3.1 Duchenne muscular dystrophy (DMD)
- 8.3.2 Spinal muscular atrophy (SMA)
- 8.4 Hemophilia A & B
- 8.5 Hepatological diseases
- 8.6 Inherited retinal disease
- 8.7 Peripheral arterial disease
- 8.8 Other indications
Chapter 9 Market Estimates and Forecast, By End Use, 2021 - 2034 ($ Mn)
- 9.1 Key trends
- 9.2 Hospitals
- 9.3 Specialty clinics and gene therapy centers
- 9.4 Academic and research institutions
- 9.5 Other End uses
Chapter 10 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Mn)
- 10.1 Key trends
- 10.2 North America
- 10.2.1 U.S.
- 10.2.2 Canada
- 10.3 Europe
- 10.3.1 Germany
- 10.3.2 UK
- 10.3.3 France
- 10.3.4 Spain
- 10.3.5 Italy
- 10.3.6 Netherlands
- 10.4 Asia Pacific
- 10.4.1 China
- 10.4.2 India
- 10.4.3 Japan
- 10.4.4 Australia
- 10.4.5 South Korea
- 10.5 Latin America
- 10.5.1 Brazil
- 10.5.2 Mexico
- 10.5.3 Argentina
- 10.6 Middle East and Africa
- 10.6.1 South Africa
- 10.6.2 Saudi Arabia
- 10.6.3 UAE
Chapter 11 Company Profiles
- 11.1 Amgen
- 11.2 Applied Genetic Technologies Corporation (AGTC)
- 11.3 Audentes Therapeutics, Inc. (Astellas Pharma)
- 11.4 Bayer
- 11.5 Beam Therapeutics
- 11.6 BioMarin Pharmaceutical
- 11.7 bluebird bio
- 11.8 Bristol-Myers Squibb Company
- 11.9 CRISPR Therapeutics
- 11.10 Gilead Sciences
- 11.11 Helixmith
- 11.12 Intellia Therapeutics
- 11.13 Krystal Biotech
- 11.14 Novartis
- 11.15 Orchard Therapeutics
- 11.16 Pfizer
- 11.17 Sangamo Therapeutics
- 11.18 Sarepta Therapeutics
- 11.19 Spark Therapeutics (Roche)
- 11.20 UniQure N.V.











