 |
市场调查报告书
商品编码
1858998
血管闭合装置市场机会、成长驱动因素、产业趋势分析及预测(2025-2034年)Vascular Closure Device Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
2024 年全球血管闭合装置市值为 17.4 亿美元,预计到 2034 年将以 5.7% 的复合年增长率成长至 30 亿美元。
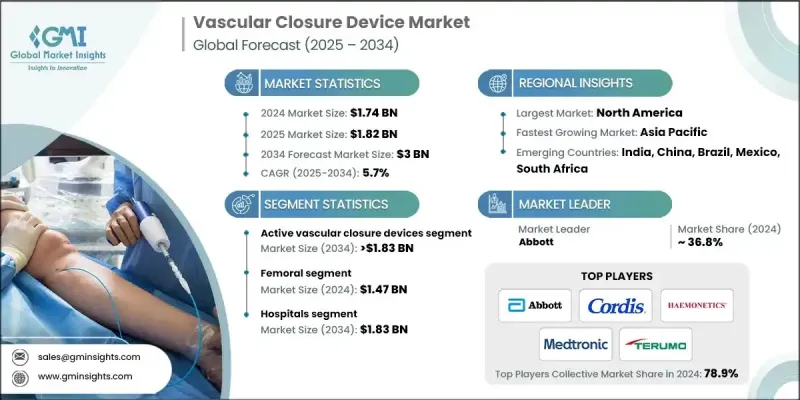
血管闭合装置市场成长可归因于多种因素,包括心血管疾病盛行率上升、介入性心臟病学和放射学手术发展,以及对门诊和当日出院手术需求的增加。血管闭合装置是重要的医疗器械,用于在导管插入术后封闭动脉穿刺口,其使用方式包括机械夹、缝线或胶原蛋白塞。与传统的止血方法(如手动压迫)相比,这些装置能够更快、更有效地止血,缩短止血时间,并最大限度地减少患者的恢復时间。随着冠状动脉疾病和心臟衰竭等心血管疾病在全球范围内的发病率上升,血管造影和介入手术的数量也随之增加,进一步推动了对血管闭合装置的需求。此外,生物可吸收闭合装置和血管外密封系统等创新技术的出现,透过解决出血和感染等併发症,扩大了血管闭合装置的应用范围,从而改善了患者预后,并加速了市场成长。
| 市场范围 | |
|---|---|
| 起始年份 | 2024 |
| 预测年份 | 2025-2034 |
| 起始值 | 17.4亿美元 |
| 预测值 | 30亿美元 |
| 复合年增长率 | 5.7% |
由于其卓越的疗效、更快的止血速度和更低的併发症发生率,主动式血管闭合装置在2024年占据了59.3%的市场份额。包括缝线型和胶原蛋白型在内的主动式血管闭合装置(VCD)因其能够闭合血管通路部位,从而加快患者恢復并提高导管介入手术的效率,越来越受到医生的青睐。预计该细分市场将继续保持领先地位,因为它能够提高导管室的效率并缩短手术时间。
2025年至2034年间,桡动脉段的复合年增长率将达4.4%。由于桡动脉入路出血併发症风险较低,且与股动脉入路相比恢復速度更快,因此正逐渐成为介入性心臟病学的首选入路。医疗机构中桡动脉手术数量的不断增加,也推动了该领域专用血管闭合装置的需求。
2024年,北美血管闭合装置市占率达44.9%。先进的医疗基础设施、尖端的导管实验室以及技术精湛的介入性心臟病专家,促进了缝合式和胶原蛋白式等先进血管闭合装置的广泛应用。微创心血管手术旨在缩短恢復时间、降低医疗成本,这一趋势进一步推动了对这些装置的需求。
全球血管闭合器材市场的主要参与者包括美敦力(Medtronic)、泰利福(Teleflex)、美瑞医疗(MERIT MEDICAL)、雅培(Abbott)、瑞克斯医疗(Rex Medical)、科迪斯(Cordis)、恩赛特血管(Ensite Vascular)、泰尔茂维尔(Terumrumo)、海蒙内特血管(ViHAa)(YEMFal特性)、医学尼科) Technologies、Vasorum、Tricol Biomedical 和 Meril。为了巩固市场地位,全球血管闭合器材市场的企业致力于提升产品创新能力并改善病患预后。许多公司正在投资研发先进的微创技术,以减少併发症并缩短恢復时间。与医疗机构和研究机构建立策略合作伙伴关係和开展合作也是扩大市场覆盖范围和获得临床验证的常见做法。
目录
第一章:方法论与范围
第二章:执行概要
第三章:行业洞察
- 产业生态系分析
- 产业影响因素
- 成长驱动因素
- 心血管疾病患者人数不断增加
- 老年人口不断增加
- 血管闭合装置的技术进步
- 介入性心臟病学和放射学手术量不断增加
- 产业陷阱与挑战
- 术后併发症风险较高
- 严格的监管环境
- 市场机会
- 拓展新兴市场
- 生物可吸收和新一代闭合装置的研发
- 成长驱动因素
- 成长潜力分析
- 报销方案
- 监管环境
- 北美洲
- 欧洲
- 亚太地区
- 技术格局
- 当前技术趋势
- 新兴技术
- 差距分析
- 波特的分析
- PESTEL 分析
- 未来市场趋势
第四章:竞争格局
- 介绍
- 公司矩阵分析
- 公司市占率分析
- 全球的
- 北美洲
- 欧洲
- 亚太地区
- 拉丁美洲
- 竞争定位矩阵
- 主要市场参与者的竞争分析
- 关键进展
- 併购
- 伙伴关係与合作
- 新设备类型发布
- 扩张计划
第五章:市场估算与预测:依设备类型划分,2021-2034年
- 主要趋势
- 主动血管闭合装置
- 胶原蛋白塞介导装置
- 缝合介导装置
- 订书钉/夹子介导的VCD
- 被动式血管闭合装置
- 止血垫/贴片
- 压缩装置
第六章:市场估算与预测:依接取量划分,2021-2034年
- 主要趋势
- 股骨
- 径向
第七章:市场估算与预测:依最终用途划分,2021-2034年
- 主要趋势
- 医院
- 门诊手术中心
- 其他最终用途
第八章:市场估算与预测:依地区划分,2021-2034年
- 主要趋势
- 北美洲
- 我们
- 加拿大
- 欧洲
- 德国
- 英国
- 法国
- 西班牙
- 义大利
- 荷兰
- 亚太地区
- 中国
- 日本
- 印度
- 澳洲
- 韩国
- 拉丁美洲
- 巴西
- 墨西哥
- 阿根廷
- 中东和非洲
- 南非
- 沙乌地阿拉伯
- 阿联酋
第九章:公司简介
- Abbott
- Cordis
- Ensite Vascular
- HAEMONETICS
- Medtronic
- Meril
- MERIT MEDICAL
- Rex Medical
- Teleflex
- TERUMO
- Transluminal Technologies
- Tricol Biomedical
- TZ Medical
- Vasorum
- Vivasure Medical
The Global Vascular Closure Device Market was valued at USD 1.74 billion in 2024 and is estimated to grow at a CAGR of 5.7% to reach USD 3 billion by 2034.

This growth can be attributed to several factors, including the rising prevalence of cardiovascular diseases, the growth of interventional cardiology and radiology procedures, and the increasing demand for outpatient and same-day discharge procedures. Vascular closure devices are essential medical tools designed to seal arterial punctures after catheterization procedures, either using mechanical clips, sutures, or collagen plugs. These devices offer quicker and more efficient hemostasis compared to traditional methods like manual compression, reducing the time needed for bleeding cessation and minimizing patient downtime. As cardiovascular diseases like coronary artery disease and heart failure rise globally, more angiographies and interventional procedures are performed, further driving the demand for vascular closure devices. Additionally, innovations such as bioabsorbable closure devices and extravascular sealing systems have expanded the adoption of VCDs by addressing complications like bleeding and infection, which improves patient outcomes and accelerates market growth.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $1.74 Billion |
| Forecast Value | $3 Billion |
| CAGR | 5.7% |
The active vascular closure devices segment held 59.3% share in 2024, owing to their superior efficacy, faster hemostasis times, and lower complication rates. Active VCDs, including suture-based and collagen-based devices, are increasingly preferred by physicians for their ability to close vascular access sites, enabling faster recovery and more efficient catheterization procedures. This segment is expected to maintain its leadership as it allows for improved cath lab performance and shorter operation times.
The radial artery segment will grow at a CAGR of 4.4% during 2025-2034. Radial access is becoming the preferred choice in interventional cardiology because it carries a lower risk of bleeding complications and allows for faster recovery compared to femoral access. The increasing number of radial artery procedures performed in healthcare settings is driving the demand for specialized closure devices in this area.
North America Vascular Closure Device Market held a 44.9% share in 2024. The presence of advanced healthcare infrastructure, cutting-edge catheterization labs, and highly skilled interventional cardiologists contributes to the widespread acceptance and use of advanced VCDs, such as suture-mediated and collagen-based devices. The trend towards minimally invasive cardiovascular procedures, designed to shorten recovery times and reduce healthcare costs, further boosts the demand for these devices.
Key players in the Global Vascular Closure Device Market include Medtronic, Teleflex, MERIT MEDICAL, Abbott, Rex Medical, Cordis, Ensite Vascular, Terumo, HAEMONETICS, Vivasure Medical, Transluminal Technologies, Vasorum, Tricol Biomedical, and Meril. To solidify their market position, companies in the Global Vascular Closure Device Market focus on enhancing product innovation and improving patient outcomes. Many companies are investing in the development of advanced, minimally invasive technologies that reduce complications and recovery times. Strategic partnerships and collaborations with healthcare providers and research institutions are also common to expand market reach and gain clinical validation.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
- 2.2 Key market trends
- 2.2.1 Regional trends
- 2.2.2 Device type trends
- 2.2.3 Access trends
- 2.2.4 End use trends
- 2.3 CXO perspectives: Strategic imperatives
- 2.3.1 Key decision points for industry executives
- 2.3.2 Critical success factors for market players
- 2.4 Future outlook and strategic recommendations
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Increasing number of patients suffering from cardiovascular diseases
- 3.2.1.2 Growing geriatric population
- 3.2.1.3 Technological advancements in vascular closure devices
- 3.2.1.4 Rising volume of interventional cardiology and radiology procedures
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 High risk associated with post-procedural complications
- 3.2.2.2 Stringent regulatory scenario
- 3.2.3 Market opportunities
- 3.2.3.1 Expansion into emerging markets
- 3.2.3.2 Development of bioresorbable and next-generation closure devices
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Reimbursement scenario
- 3.5 Regulatory landscape
- 3.5.1 North America
- 3.5.2 Europe
- 3.5.3 Asia Pacific
- 3.6 Technology landscape
- 3.6.1 Current technological trends
- 3.6.2 Emerging technologies
- 3.7 Vascular closure device market, 2021-2034 (Units)
- 3.7.1 North America
- 3.7.2 Europe
- 3.7.3 Asia Pacific
- 3.7.4 Latin America
- 3.7.5 Middle East and Africa
- 3.8 Gap analysis
- 3.9 Porter's analysis
- 3.10 PESTEL analysis
- 3.11 Future market trends
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company matrix analysis
- 4.3 Company market share analysis
- 4.3.1 Global
- 4.3.2 North America
- 4.3.3 Europe
- 4.3.4 Asia Pacific
- 4.3.5 LAMEA
- 4.4 Competitive positioning matrix
- 4.5 Competitive analysis of major market players
- 4.6 Key developments
- 4.6.1 Mergers and acquisitions
- 4.6.2 Partnerships and collaborations
- 4.6.3 New device type launches
- 4.6.4 Expansion plans
Chapter 5 Market Estimates and Forecast, By Device Type, 2021 - 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Active vascular closure devices
- 5.2.1 Collagen plug mediated device
- 5.2.2 Suture-mediated devices
- 5.2.3 Staple/clip-mediated VCD
- 5.3 Passive vascular closure devices
- 5.3.1 Haemostasis pads/patches
- 5.3.2 Compression devices
Chapter 6 Market Estimates and Forecast, By Access, 2021 - 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Femoral
- 6.3 Radial
Chapter 7 Market Estimates and Forecast, By End Use, 2021 - 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Hospitals
- 7.3 Ambulatory surgical centers
- 7.4 Other end use
Chapter 8 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Mn)
- 8.1 Key trends
- 8.2 North America
- 8.2.1 U.S.
- 8.2.2 Canada
- 8.3 Europe
- 8.3.1 Germany
- 8.3.2 UK
- 8.3.3 France
- 8.3.4 Spain
- 8.3.5 Italy
- 8.3.6 Netherlands
- 8.4 Asia Pacific
- 8.4.1 China
- 8.4.2 Japan
- 8.4.3 India
- 8.4.4 Australia
- 8.4.5 South Korea
- 8.5 Latin America
- 8.5.1 Brazil
- 8.5.2 Mexico
- 8.5.3 Argentina
- 8.6 Middle East and Africa
- 8.6.1 South Africa
- 8.6.2 Saudi Arabia
- 8.6.3 UAE
Chapter 9 Company Profiles
- 9.1 Abbott
- 9.2 Cordis
- 9.3 Ensite Vascular
- 9.4 HAEMONETICS
- 9.5 Medtronic
- 9.6 Meril
- 9.7 MERIT MEDICAL
- 9.8 Rex Medical
- 9.9 Teleflex
- 9.10 TERUMO
- 9.11 Transluminal Technologies
- 9.12 Tricol Biomedical
- 9.13 TZ Medical
- 9.14 Vasorum
- 9.15 Vivasure Medical












