 |
市场调查报告书
商品编码
1871275
生物分析检测服务市场机会、成长驱动因素、产业趋势分析及预测(2025-2034年)Bioanalytical Testing Services Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
2024 年全球生物分析测试服务市场价值为 42 亿美元,预计到 2034 年将以 8.2% 的复合年增长率增长至 93 亿美元。
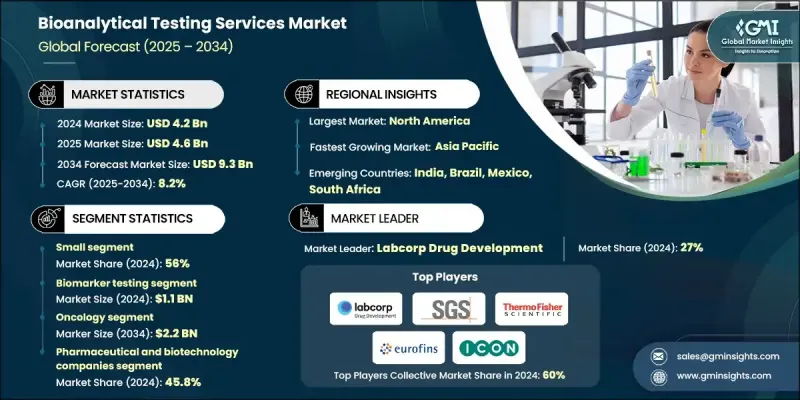
实验室检测外包趋势的日益增长、生物分析技术的快速发展以及药物研发和审批流程的不断增加,共同推动了生物分析检测服务的稳定成长。临床试验和研究活动的激增也促进了市场扩张。生物分析检测服务涉及对包括血液、组织和尿液在内的生物样本进行分析,以定量生物基质中的药物、代谢物、生物标记和其他分析物。这些服务对于製药和生物技术产业进行药物动力学研究、生物等效性评估、方法验证和临床试验支援至关重要。它们确保药物的安全性、有效性和合规性,同时为药效学研究和生物标记分析提供关键资料。对精确、可靠和高品质生物分析资料的需求不断增长,并持续推动全球对这些服务的应用。
| 市场范围 | |
|---|---|
| 起始年份 | 2024 |
| 预测年份 | 2025-2034 |
| 起始值 | 42亿美元 |
| 预测值 | 93亿美元 |
| 复合年增长率 | 8.2% |
到2024年,小分子药物市占率将达到56%。小分子药物在各个治疗领域的持续普及推动了对小分子药物测试需求的成长。小分子药物结构相对简单、监管途径成熟且生产成本低廉,因此深受製药公司青睐。此外,仿製药获批数量的激增以及品牌小分子药物专利的到期也加剧了生物等效性研究的需求。
预计到2024年,製药和生物技术公司将占据45.8%的市场。这些公司高度依赖生物分析检测来支援复杂生物製剂、生物相似药和标靶疗法的研发,从而确保符合监管要求并确保资料的完整性。生物分析服务提供者与製药或生技公司之间的合作正在迅速扩展,这不仅提高了营运效率,加快了药物研发进程,也维持了高品质的检测标准。
2024年,美国生物分析检测服务市场规模预估达15亿美元。推动市场成长的主要因素包括製药和生物技术公司研发投入的增加,尤其是在生物製剂开发领域,以及与合约研究组织(CRO)和合约开发与生产组织(CDMO)的策略合作。北美生物分析检测服务市场受益于先进的製药和生物技术基础设施、广泛的研究活动以及大量需要专业检测服务的药物发现和开发项目。
全球生物分析检测服务市场的主要公司包括:赛默飞世尔科技公司 (Thermo Fisher Scientific Inc.)、Intertek Group Plc、药明康德 (WuXi AppTec)、ICON Plc、Pace Analytical Services LLC、BioAgilytix、Eurofins Scientific、Labcorp Drug、Face Analytical Services LLC、BioAgilytix、Eurofins Scientific、Labcorp Drug Development、Charles Riverynea. Generals. Health、Cambrex Corporation、Altasciences 和 LGC Limited。这些公司正透过多种策略倡议巩固其市场地位。它们大力投资研发,以提升分析能力、提高灵敏度并扩大服务范围。与製药、生物技术和合约研究机构的策略合作与伙伴关係,有助于加速市场拓展并提升服务效率。併购则有助于整合技术专长、扩大地域覆盖范围并扩大营运规模。此外,各公司也致力于获得监管部门的批准、认证和方法验证,以提升信誉并吸引高端客户。
目录
第一章:方法论与范围
第二章:执行概要
第三章:行业洞察
- 产业生态系分析
- 产业影响因素
- 成长驱动因素
- 实验室检测服务外包趋势日益增长
- 生物分析技术的不断进步
- 药物研发和审批流程的不断发展
- 正在进行的研究活动和临床试验数量不断增加
- 产业陷阱与挑战
- 实验室维护的复杂监管框架
- 先进分析仪器和技术成本高昂
- 市场机会
- 扩大新兴市场的临床试验
- 人工智慧与自动化在生物分析工作流程中的集成
- 成长驱动因素
- 成长潜力分析
- 监管环境
- 技术进步
- 当前技术趋势
- 新兴技术
- 供应链分析
- 报销方案
- 2024年定价分析
- 未来市场趋势
- 差距分析
- 波特的分析
- PESTEL 分析
第四章:竞争格局
- 介绍
- 公司市占率分析
- 公司矩阵分析
- 主要市场参与者的竞争分析
- 竞争定位矩阵
- 关键进展
- 併购
- 伙伴关係与合作
- 新产品发布
- 扩张计划
第五章:市场估算与预测:依分子类型划分,2021-2034年
- 主要趋势
- 小分子
- 大分子
第六章:市场估算与预测:依测试类型划分,2021-2034年
- 主要趋势
- DMPK 检测
- 生物标记检测
- 病毒学检测
- 体内病毒学检测
- 体外病毒学检测
- 血清学检测
- 免疫原性检测
- 其他测试类型
第七章:市场估计与预测:依应用领域划分,2021-2034年
- 主要趋势
- 肿瘤学
- 神经病学
- 传染病
- 胃肠病学
- 心臟病学
- 其他应用
第八章:市场估算与预测:依最终用途划分,2021-2034年
- 主要趋势
- 製药和生物技术公司
- 合约研究机构
- 研究和学术机构
- 其他最终用途
第九章:市场估计与预测:依地区划分,2021-2034年
- 主要趋势
- 北美洲
- 我们
- 加拿大
- 欧洲
- 德国
- 英国
- 法国
- 西班牙
- 义大利
- 荷兰
- 亚太地区
- 中国
- 印度
- 日本
- 澳洲
- 韩国
- 拉丁美洲
- 巴西
- 墨西哥
- 阿根廷
- 中东和非洲
- 沙乌地阿拉伯
- 南非
- 阿联酋
第十章:公司简介
- Altasciences
- BioAgilytix
- Cambrex Corporation
- Charles River Laboratories, Inc.
- Eurofins Scientific
- Frontage Labs
- ICON Plc
- Intertek Group Plc
- Labcorp Drug Development
- LGC Limited
- Pace Analytical Services LLC
- SGS Societe Generale de Surveillance SA
- Syneos Health
- Thermo Fisher Scientific Inc.
- WuXi AppTec
The Global Bioanalytical Testing Services Market was valued at USD 4.2 billion in 2024 and is estimated to grow at a CAGR of 8.2% to reach USD 9.3 billion by 2034.

The steady growth is fueled by the increasing trend of outsourcing laboratory testing, rapid advancements in bioanalytical technologies, and the rising volume of drug development and approval processes. The surge in clinical trials and research activities also supports market expansion. Bioanalytical testing services involve analyzing biological samples, including blood, tissue, and urine, to quantify drugs, metabolites, biomarkers, and other analytes in biological matrices. These services are crucial for the pharmaceutical and biotechnology industries to conduct pharmacokinetic studies, bioequivalence evaluations, method validations, and clinical trial support. They ensure drug safety, efficacy, and regulatory compliance while providing essential data for pharmacodynamic studies and biomarker analysis. Growing demand for precise, reliable, and high-quality bioanalytical data continues to drive the adoption of these services globally.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $4.2 Billion |
| Forecast Value | $9.3 Billion |
| CAGR | 8.2% |
The small molecule segment held a 56% share in 2024. The increasing demand for small molecule testing is driven by the continued prevalence of small molecule drugs in various therapeutic areas. Their relatively simpler chemical structures, established regulatory pathways, and cost-effective production make them favorable for pharmaceutical companies. Moreover, the surge in generic drug approvals and patent expirations for branded small-molecule drugs has escalated the need for bioequivalence studies.
The pharmaceutical and biotechnology companies segment held a 45.8% share in 2024. These organizations rely heavily on bioanalytical testing to support the development of complex biologics, biosimilars, and targeted therapies, ensuring regulatory compliance and robust data integrity. Collaborations between bioanalytical service providers and pharmaceutical or biotech companies are expanding rapidly, enhancing operational efficiency, accelerating drug development timelines, and maintaining high-quality testing standards.
United States Bioanalytical Testing Services Market was valued at USD 1.5 billion in 2024. Growth is driven by increased R&D investments by pharmaceutical and biotechnology firms, particularly in biologics development, and strategic partnerships with contract research organizations (CROs) and contract development and manufacturing organizations (CDMOs). North America's bioanalytical testing services market benefits from advanced pharmaceutical and biotechnology infrastructure, extensive research activities, and a high number of drug discovery and development projects requiring specialized testing services.
Key companies in the Global Bioanalytical Testing Services Market include Thermo Fisher Scientific Inc., Intertek Group Plc, WuXi AppTec, ICON Plc, Pace Analytical Services LLC, BioAgilytix, Eurofins Scientific, Labcorp Drug Development, Charles River Laboratories, Inc., SGS Societe Generale de Surveillance SA, Frontage Labs, Syneos Health, Cambrex Corporation, Altasciences, and LGC Limited. Companies in the Global Bioanalytical Testing Services Market are strengthening their presence through several strategic approaches. They are investing heavily in research and development to enhance analytical capabilities, improve sensitivity, and expand service portfolios. Strategic collaborations and partnerships with pharmaceutical, biotechnology, and contract research organizations accelerate market reach and improve service efficiency. Mergers and acquisitions help consolidate technological expertise, expand geographical presence, and scale operations. Firms are also focusing on regulatory approvals, accreditation, and method validation to enhance credibility and attract high-profile clients.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definition
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/Country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 360° synopsis
- 2.2 Key market trends
- 2.2.1 Regional trends
- 2.2.2 Molecule type trends
- 2.2.3 Test type trend
- 2.2.4 Application trends
- 2.2.5 End use trends
- 2.3 CXO perspectives: Strategic imperatives
- 2.3.1 Key decision points for industry executives
- 2.3.2 Critical success factors for market players
- 2.4 Future outlook and strategic recommendations
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Increased trend of outsourcing laboratory testing services
- 3.2.1.2 Rising advancements in bioanalytical technology
- 3.2.1.3 Growing drug development and approval processes
- 3.2.1.4 Increasing volume of ongoing research activities and clinical trials
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 Complex regulatory framework for maintaining laboratories
- 3.2.2.2 High cost of advanced analytical instruments and technologies
- 3.2.3 Market opportunities
- 3.2.3.1 Expansion of clinical trials in emerging markets
- 3.2.3.2 Integration of AI and automation in bioanalytical workflows
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.5 Technological advancements
- 3.5.1 Current technological trends
- 3.5.2 Emerging technologies
- 3.6 Supply chain analysis
- 3.7 Reimbursement scenario
- 3.8 Pricing analysis, 2024
- 3.9 Future market trends
- 3.10 Gap analysis
- 3.11 Porter's analysis
- 3.12 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.3 Company matrix analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Key developments
- 4.6.1 Mergers and acquisitions
- 4.6.2 Partnerships and collaborations
- 4.6.3 New product launches
- 4.6.4 Expansion plans
Chapter 5 Market Estimates and Forecast, By Molecule Type, 2021 - 2034 ($ Bn)
- 5.1 Key trends
- 5.2 Small molecule
- 5.3 Large molecule
Chapter 6 Market Estimates and Forecast, By Test Type, 2021 - 2034 ($ Bn)
- 6.1 Key trends
- 6.2 DMPK Testing
- 6.3 Biomarker testing
- 6.4 Virology testing
- 6.4.1 In-vivo virology testing
- 6.4.2 In-vitro virology testing
- 6.5 Serology testing
- 6.6 Immunogenicity testing
- 6.7 Other test types
Chapter 7 Market Estimates and Forecast, By Application, 2021 - 2034 ($ Bn)
- 7.1 Key trends
- 7.2 Oncology
- 7.3 Neurology
- 7.4 Infectious diseases
- 7.5 Gastroenterology
- 7.6 Cardiology
- 7.7 Other applications
Chapter 8 Market Estimates and Forecast, By End Use, 2021 - 2034 ($ Bn)
- 8.1 Key trends
- 8.2 Pharmaceutical and biotechnology companies
- 8.3 Contract research organizations
- 8.4 Research and academic institutes
- 8.5 Other end use
Chapter 9 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Bn)
- 9.1 Key trends
- 9.2 North America
- 9.2.1 U.S.
- 9.2.2 Canada
- 9.3 Europe
- 9.3.1 Germany
- 9.3.2 UK
- 9.3.3 France
- 9.3.4 Spain
- 9.3.5 Italy
- 9.3.6 Netherlands
- 9.4 Asia Pacific
- 9.4.1 China
- 9.4.2 India
- 9.4.3 Japan
- 9.4.4 Australia
- 9.4.5 South Korea
- 9.5 Latin America
- 9.5.1 Brazil
- 9.5.2 Mexico
- 9.5.3 Argentina
- 9.6 Middle East and Africa
- 9.6.1 Saudi Arabia
- 9.6.2 South Africa
- 9.6.3 UAE
Chapter 10 Company Profiles
- 10.1 Altasciences
- 10.2 BioAgilytix
- 10.3 Cambrex Corporation
- 10.4 Charles River Laboratories, Inc.
- 10.5 Eurofins Scientific
- 10.6 Frontage Labs
- 10.7 ICON Plc
- 10.8 Intertek Group Plc
- 10.9 Labcorp Drug Development
- 10.10 LGC Limited
- 10.11 Pace Analytical Services LLC
- 10.12 SGS Societe Generale de Surveillance SA
- 10.13 Syneos Health
- 10.14 Thermo Fisher Scientific Inc.
- 10.15 WuXi AppTec










