 |
市场调查报告书
商品编码
1871303
即时检测市场机会、成长驱动因素、产业趋势分析及预测(2025-2034年)Point of Care Testing Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
2024 年全球即时检测市场价值为 420 亿美元,预计到 2034 年将以 7% 的复合年增长率增长至 820 亿美元。
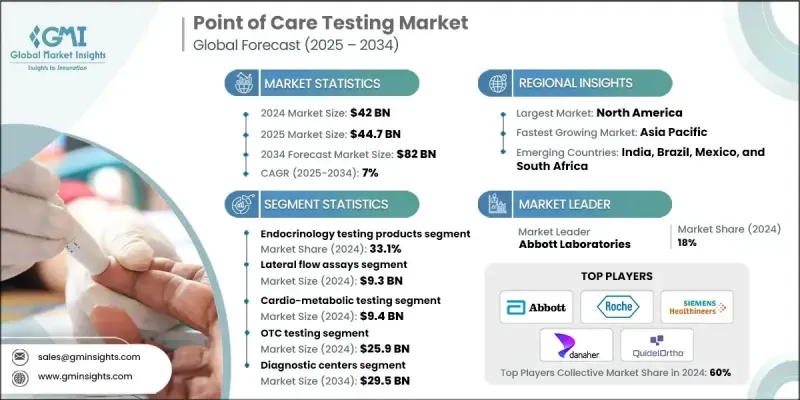
市场扩张的驱动因素包括发展中国家疾病盛行率的上升、配备先进检测技术的诊断实验室数量的增加以及研发方面的大量投资。急诊和偏远地区对快速、准确诊断的需求不断增长,促使各国政府和医疗机构采用创新的即时检测(POC)解决方案。即时检测使临床医生能够在患者所在之处直接获得快速、实验室层级的检测结果,有助于快速做出医疗决策并减少对中心实验室的依赖。这些设备采用微流控平台、免疫分析和侧向流动检测等先进技术来分析血液、尿液或唾液,通常可在几分钟内提供结果。现代设备通常与行动应用程式或电子健康记录集成,从而提高了农村和医疗服务不足地区的可及性。
| 市场范围 | |
|---|---|
| 起始年份 | 2024 |
| 预测年份 | 2025-2034 |
| 起始值 | 420亿美元 |
| 预测值 | 820亿美元 |
| 复合年增长率 | 7% |
2024年,内分泌检测产品市占率高达33.1%。由于糖尿病和甲状腺疾病等内分泌疾病的盛行率不断上升,对快速且准确诊断的需求日益增长,该细分市场正稳步发展。生物感测器、免疫分析和微流控技术的进步提高了检测效率和准确性。血糖仪和糖化血红蛋白分析仪等设备正逐渐发展成为智慧工具,并可透过行动应用程式进行远端监测和远距医疗应用。
2024年,侧向流动检测(LFA)市场规模预计将达93亿美元。 LFA是一种纸基诊断设备,利用抗体检测液体样本中的目标物质。由于其操作简单、快速且高效,LFA被广泛应用。传染病发生率的上升加速了对LFA的需求,LFA使医疗服务提供者能够快速筛检、诊断和应对患者,从而支持更快速的治疗决策和更好的治疗效果。
2024年,美国即时检测市场规模预计将达125亿美元。推动市场成长的主要因素包括疾病盛行率的上升(尤其是糖尿病)以及快速、技术驱动诊断解决方案的日益普及。美国医疗保健产业对创新和可近性的重视,使其成为即时检测技术的领先市场。
全球即时检测市场的主要参与者包括 Meridian Bioscience、Abbott Laboratories、Bio-Rad Laboratories、LifeScan IP Holdings, LLC、Acon Laboratories、Becton, Dickinson and Company、Danaher Corporation、BioMerieux SA、Dexcom, Inc.、QuidelOrtron Corporation、Dragerageral AG & Co. Kr. Biomedical、Siemens Healthineers AG 和 Sysmex Corporation。这些企业正利用多种策略来巩固其市场地位。他们大力投资研发,以开发速度更快、更精准、更互联的诊断设备。策略合作、伙伴关係和收购使企业能够扩展产品组合併进入新的区域市场。许多企业专注于技术创新,将人工智慧、互联技术和数位健康平台整合到设备中,以改善资料管理和远距医疗能力。
目录
第一章:方法论与范围
第二章:执行概要
第三章:行业洞察
- 产业生态系分析
- 产业影响因素
- 成长驱动因素
- 发展中国家疾病盛行率呈上升趋势
- 配备先进诊断设备的病理实验室和服务机构数量激增
- 即时检测技术的进步
- 增加研发投入
- 产业陷阱与挑战
- 严格的监管框架
- 产品开发成本高
- 市场机会
- 偏远和农村地区的需求不断增长
- 与数位健康平台集成
- 成长驱动因素
- 成长潜力分析
- 监管环境
- 北美洲
- 亚太地区
- 欧洲
- 技术格局
- 未来市场趋势
- 差距分析
- 波特的分析
- PESTEL 分析
第四章:竞争格局
- 介绍
- 公司市占率分析
- 公司矩阵分析
- 主要市场参与者的竞争分析
- 竞争定位矩阵
- 战略仪錶板
- 关键进展
- 併购
- 伙伴关係与合作
- 新产品发布
- 扩张计划
第五章:市场估算与预测:依产品划分,2021-2034年
- 主要趋势
- 内分泌检测产品
- 血糖监测
- 条状物
- 仪表
- 柳叶刀
- 胆固醇检测产品
- 验孕产品
- 生育力检测产品
- 甲状腺功能检查
- 血糖监测
- 心血管代谢检测产品
- 心臟标记检测产品
- 高敏感性肌钙蛋白I (hsTnI)
- BNP(B型钠尿肽)
- D-二聚体
- CK-MB(肌酸激酶-MB)
- 肌红蛋白
- 其他心臟标记检测
- 血气(肺功能)
- 代谢物检测产品
- 电解质测试
- 肝功能
- 胆红素
- 丙胺酸转氨酶(ALT)
- 肾功能
- 肌酸酐
- 尿素
- 尿酸
- HBA1C 检测产品
- 心臟标记检测产品
- 传染病检测产品
- 流感检测产品
- HIV检测产品
- 丙型肝炎检测产品
- 性传染病(STD)检测产品
- 医疗相关感染 (HAI) 检测产品
- 呼吸道感染检测产品
- 热带疾病检测产品
- 其他传染病检测产品
- 凝血检测产品
- PT/INR 检测产品
- 活化凝血时间 (ACT/APTT) 检测产品
- 肿瘤/癌症标记检测产品
- 尿液分析检测产品
- 血液学检测产品
- 滥用药物(DoA)检测产品
- 粪便隐血试验产品
- 其他产品
第六章:市场估计与预测:依技术划分,2021-2034年
- 主要趋势
- 侧向流动检测
- 油尺
- 微流控技术
- 分子诊断
- 免疫测定
- 凝集试验
- 流通式
- 固相
- 生物感测器
- 电化学
- 光学的
- 热的
- 品质敏感型
- 其他生物感测器
第七章:市场估计与预测:依应用领域划分,2021-2034年
- 主要趋势
- 心血管代谢测试
- 传染病检测
- 肾臟科检查
- 药物滥用检测
- 血糖检测
- 妊娠测试
- 癌症生物标记检测
- 其他应用
第八章:市场估算与预测:以处方量计算,2021-2034年
- 主要趋势
- 非处方药检测
- 基于处方的检测
第九章:市场估算与预测:依最终用途划分,2021-2034年
- 主要趋势
- 医院
- 诊断中心
- 研究实验室
- 居家照护环境
- 其他最终用途
第十章:市场估计与预测:依地区划分,2021-2034年
- 主要趋势
- 北美洲
- 我们
- 加拿大
- 欧洲
- 德国
- 英国
- 法国
- 西班牙
- 义大利
- 荷兰
- 亚太地区
- 中国
- 日本
- 印度
- 澳洲
- 韩国
- 拉丁美洲
- 巴西
- 墨西哥
- 阿根廷
- 中东和非洲
- 南非
- 沙乌地阿拉伯
- 阿联酋
第十一章:公司简介
- Abbott Laboratories
- Acon Laboratories
- Becton, Dickinson, and Company
- BioMerieux SA
- Bio-Rad Laboratories, Inc.
- Danaher Corporation
- Dexcom, Inc
- Dragerwerk AG & Co. KGaA
- F. Hoffmann-La Roche Ltd.
- LifeScan IP Holdings, LLC
- Medtronic Plc
- Meridian Bioscience, Inc.
- Nova Biomedical
- QuidelOrtho Corporation
- Siemens Healthineers AG
- Sysmex Corporation
The Global Point of Care Testing Market was valued at USD 42 billion in 2024 and is estimated to grow at a CAGR of 7% to reach USD 82 billion by 2034.

The market's expansion is driven by increasing disease prevalence in developing nations, the growing number of diagnostic laboratories equipped with advanced testing technologies, and significant investments in research and development. Rising demand for rapid, accurate diagnostics in emergency care and remote locations is pushing governments and healthcare organizations to adopt innovative POC testing solutions. Point-of-care testing enables clinicians to obtain fast, lab-grade results directly at the patient's location, facilitating quick medical decisions and reducing dependence on central labs. Devices use advanced technologies such as microfluidic platforms, immunoassays, and lateral flow assays to analyze blood, urine, or saliva, often providing results within minutes. Modern devices often integrate with mobile apps or electronic health records, improving accessibility in rural and underserved areas.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $42 Billion |
| Forecast Value | $82 Billion |
| CAGR | 7% |
The endocrinology testing products segment held a substantial share of 33.1% in 2024. This segment is growing steadily due to the increasing prevalence of endocrine disorders, including diabetes and thyroid-related conditions, which demand rapid and accurate diagnostics. Advances in biosensors, immunoassays, and microfluidic technology have improved test efficiency and accuracy. Devices like glucose meters and HbA1c analyzers are evolving into smart tools connected to mobile apps for remote monitoring and telehealth applications.
The lateral flow assays segment was valued at USD 9.3 billion in 2024. LFAs are paper-based diagnostic devices that detect target substances in liquid samples using antibodies. They are widely used due to their simplicity, speed, and ease of use. Rising incidences of infectious diseases have accelerated the demand for LFAs, which allow healthcare providers to quickly screen, diagnose, and respond to patients, supporting faster treatment decisions and better outcomes.
U.S. Point of Care Testing Market was valued at USD 12.5 billion in 2024. Growth is being driven by increasing disease prevalence, particularly diabetes, and the rising adoption of fast, technology-enabled diagnostic solutions. The U.S. healthcare sector's focus on innovation and accessibility has positioned it as a leading market for point-of-care testing technologies.
Key players in the Global Point of Care Testing Market include Meridian Bioscience, Abbott Laboratories, Bio-Rad Laboratories, LifeScan IP Holdings, LLC, Acon Laboratories, Becton, Dickinson, and Company, Danaher Corporation, BioMerieux SA, Dexcom, Inc., QuidelOrtho Corporation, Dragerwerk AG & Co. KGaA, F. Hoffmann-La Roche Ltd., Medtronic Plc, Nova Biomedical, Siemens Healthineers AG, and Sysmex Corporation. Companies in the Point of Care Testing Market are leveraging multiple strategies to strengthen their market presence. They are heavily investing in R&D to develop faster, more accurate, and connected diagnostic devices. Strategic collaborations, partnerships, and acquisitions allow firms to expand product portfolios and enter new regional markets. Many are focusing on technological innovation, integrating AI, connectivity, and digital health platforms into devices to improve data management and telemedicine capabilities.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/Country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
- 2.2 Key market trends
- 2.2.1 Regional trends
- 2.2.2 Products trends
- 2.2.3 Technology trends
- 2.2.4 Prescription trends
- 2.2.5 Application trends
- 2.2.6 End use trends
- 2.3 CXO perspectives: Strategic imperatives
- 2.3.1 Key decision points for industry executives
- 2.3.2 Critical success factors for market players
- 2.4 Future outlook and strategic recommendations
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Upward trend in disease prevalence among developing countries
- 3.2.1.2 Surging number of pathology labs and services equipped with advanced diagnostic equipment
- 3.2.1.3 Technological advancements in point of care tests
- 3.2.1.4 Increasing research and development investment
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 Stringent regulatory framework
- 3.2.2.2 High cost of product development
- 3.2.3 Market opportunities
- 3.2.3.1 Growing demand in remote and rural areas
- 3.2.3.2 Integration with digital health platforms
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.4.1 North America
- 3.4.2 Asia Pacific
- 3.4.3 Europe
- 3.5 Technology landscape
- 3.6 Future market trends
- 3.7 Gap analysis
- 3.8 Porter's analysis
- 3.9 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.3 Company matrix analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Strategy dashboard
- 4.7 Key developments
- 4.7.1 Mergers and acquisitions
- 4.7.2 Partnerships and collaborations
- 4.7.3 New product launches
- 4.7.4 Expansion plans
Chapter 5 Market Estimates and Forecast, By Product, 2021 - 2034 ($ Bn)
- 5.1 Key trends
- 5.2 Endocrinology testing products
- 5.2.1 Glucose monitoring
- 5.2.1.1 Strips
- 5.2.1.2 Meters
- 5.2.1.3 Lancets
- 5.2.2 Cholesterol testing products
- 5.2.3 Pregnancy testing products
- 5.2.4 Fertility testing products
- 5.2.5 Thyroid function tests
- 5.2.1 Glucose monitoring
- 5.3 Cardiometabolic testing products
- 5.3.1 Cardiac marker testing products
- 5.3.1.1 hsTnI (High-Sensitivity Troponin I)
- 5.3.1.2 BNP (B-Type Natriuretic Peptide)
- 5.3.1.3 D-dimer
- 5.3.1.4 CK-MB (Creatine Kinase-MB)
- 5.3.1.5 Myoglobin
- 5.3.1.6 Other Cardiac Marker Testing
- 5.3.2 Blood gas (Lung function)
- 5.3.3 Metabolite testing products
- 5.3.3.1 Electrolytes testing
- 5.3.3.2 Liver function
- 5.3.3.2.1 Bilirubin
- 5.3.3.2.2 Alanine transaminase (ALT)
- 5.3.3.3 Kidney function
- 5.3.3.3.1 Creatinine
- 5.3.3.3.2 Urea
- 5.3.3.3.3 Uric Acid
- 5.3.4 HBA1C testing products
- 5.3.1 Cardiac marker testing products
- 5.4 Infectious disease testing products
- 5.4.1 Influenza testing products
- 5.4.2 HIV testing products
- 5.4.3 Hepatitis C testing products
- 5.4.4 Sexually transmitted disease (STD) testing products
- 5.4.5 Healthcare-associated infection (HAI) testing products
- 5.4.6 Respiratory infection testing products
- 5.4.7 Tropical disease testing products
- 5.4.8 Other infectious disease testing products
- 5.5 Coagulation testing products
- 5.5.1 PT/INR testing products
- 5.5.2 Activated clotting time (ACT/APTT) testing products
- 5.6 Tumor/cancer marker testing products
- 5.7 Urinalysis testing products
- 5.8 Hematology testing products
- 5.9 Drug-of-abuse (DoA) testing products
- 5.10 Fecal occult testing products
- 5.11 Other products
Chapter 6 Market Estimates and Forecast, By Technology, 2021 - 2034 ($ Bn)
- 6.1 Key trends
- 6.2 Lateral flow assays
- 6.3 Dipsticks
- 6.4 Microfluidics
- 6.5 Molecular diagnostics
- 6.6 Immunoassays
- 6.7 Agglutination assays
- 6.8 Flow-through
- 6.9 Solid phase
- 6.10 Biosensors
- 6.10.1 Electrochemical
- 6.10.2 Optical
- 6.10.3 Thermal
- 6.10.4 Mass-sensitive
- 6.10.5 Other biosensors
Chapter 7 Market Estimates and Forecast, By Application, 2021 - 2034 ($ Bn)
- 7.1 Key trends
- 7.2 Cardio metabolic testing
- 7.3 Infectious disease testing
- 7.4 Nephrology testing
- 7.5 Drug-of-abuse (DoA) testing
- 7.6 Blood glucose testing
- 7.7 Pregnancy testing
- 7.8 Cancer biomarker testing
- 7.9 Other applications
Chapter 8 Market Estimates and Forecast, By Prescription, 2021 - 2034 ($ Bn)
- 8.1 Key trends
- 8.2 OTC testing
- 8.3 Prescription-based testing
Chapter 9 Market Estimates and Forecast, By End Use, 2021 - 2034 ($ Bn)
- 9.1 Key trends
- 9.2 Hospitals
- 9.3 Diagnostic centers
- 9.4 Research laboratories
- 9.5 Home-care settings
- 9.6 Other end use
Chapter 10 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Bn)
- 10.1 Key trends
- 10.2 North America
- 1.1.1 U.S.
- 1.1.2 Canada
- 10.3 Europe
- 1.1.3 Germany
- 1.1.4 UK
- 1.1.5 France
- 1.1.6 Spain
- 1.1.7 Italy
- 1.1.8 Netherlands
- 10.4 Asia Pacific
- 1.1.9 China
- 1.1.10 Japan
- 1.1.11 India
- 1.1.12 Australia
- 1.1.13 South Korea
- 10.5 Latin America
- 1.1.14 Brazil
- 1.1.15 Mexico
- 1.1.16 Argentina
- 10.6 Middle East and Africa
- 1.1.17 South Africa
- 1.1.18 Saudi Arabia
- 1.1.19 UAE
Chapter 11 Company Profiles
- 11.1 Abbott Laboratories
- 11.2 Acon Laboratories
- 11.3 Becton, Dickinson, and Company
- 11.4 BioMerieux SA
- 11.5 Bio-Rad Laboratories, Inc.
- 11.6 Danaher Corporation
- 11.7 Dexcom, Inc
- 11.8 Dragerwerk AG & Co. KGaA
- 11.9 F. Hoffmann-La Roche Ltd.
- 11.10 LifeScan IP Holdings, LLC
- 11.11 Medtronic Plc
- 11.12 Meridian Bioscience, Inc.
- 11.13 Nova Biomedical
- 11.14 QuidelOrtho Corporation
- 11.15 Siemens Healthineers AG
- 11.16 Sysmex Corporation








