 |
市场调查报告书
商品编码
1186912
口服固体製剂 (OSDF):心血管市场Oral Solid Dosage Forms - Cardiology Markets |
||||||
FDA 批准的高血压药物 API
示例视图
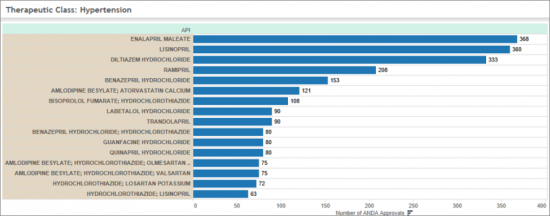
OSDF 用于高血压适应症的药物占心血管领域所有已批准 API 的 90% 以上。 该组包括主要处方药,例如:
|
|
示例屏幕

本报告考察了心血管适应症口服固体製剂 (OSDF) 市场,包括按供应商、主要产品和药物类别分类的详细数据。
内容
- 心血管口服固体剂型 (OSDF)
- 执行摘要
- 一般 SDF:产品注意事项
- 常见的固体剂型:心血管适应症
- 高血压
- 心血管治疗类别:除高血压外的所有类别
- 高血压适应症 OSDF 药物的主要供应商
- 药品类别:供应商的市场占有率
- 药物类别:ACE 抑製剂(Abbvie、Graviti Pharms 等)
- 药物类别:Ace 抑製剂(Graviti Pharms、Zydus Pharma USA 等)
- 药物类别:肾上腺素受体阻滞剂(Able、Mylan 等)
- 药物类别:肾上腺素受体阻滞剂(Mylan、Zydus Pharms 等)
- 药物类别:血管紧张素 II 受体(Ajanta Pharma Ltd、Zydus Pharms 等)
- 药物类别:钙通道阻滞剂(Accord Healthcare、Mutual Pharm 等)
- 药物类别:钙通道阻滞剂(Mylan、Zydus Pharms 等)
- 药物类别:利尿剂(Actavis、Monarch Pharms 等)
- 药物类别:利尿剂(Actavis、Monarch Pharms 等)
- 药物类别:血管扩张剂 (Actavis/Woodward)
- 心血管适应症的 OSDF 供应商
- Accord Healthcare
- Actavis
- Alembic
- Amneal Pharms
- ANI Pharms
- Apotex
- Aurobindo
- Dr. Reddy's
- Glenmark
- Hikma
- Lupin
- Mylan
- Sandoz
- Sun Pharma
- Teva
- Torrent Pharms
- Watson Labs
- Zydus Pharms USA
- OSDF-剂量图
Introduction
Greystone Research is pleased to announce the publication of a new life science information resource, a comprehensive overview of the markets, products and participants providing the driving force behind the OSDF segment of the drug delivery sector. The report has been designed and developed to provide pharmaceutical company decision makers, drug developers and formulators, drug device designers, and industry strategists with a detailed understanding of the expanding impact of Ora; Solid Dosage Forms on therapies, pharmaceutical strategies and healthcare treatment protocols. Provider organization business managers, healthcare administrators and investors will also benefit from this publication.
Generating Product Improvements in a Mature Market
The generic solid dosage form drug segment is a rapidly evolving and highly unpredictable environment that has been a challenge to decision makers attempting to map strategies for success in this segment. There are 600 distinct APIs for which there is at least one approved generic solid dosage form ANDA. These APIs account for over four thousand approved ANDAs. Including all approved doses, the current universe of generic solid dosage forms consists of thousands of unique tablet and capsule products. These products are marketed and supplied by more than 800 companies. Competition among the various drug and therapeutic classes is uneven, with the top ten segments accounting for a disproportionate level of activity and revenue. Understanding the underlying factors affecting business performance is key to attaining financial targets.
FDA Approved Hypertension APIs
SAMPLE VIEW

OSDF drugs indicated for Hypertension represent more than 90 % of all approved APIs in the Cardiology market segment. This group includes a number of well-known prescription medicines, including:
|
|
Other non-hypertensive drugs approved for Cardiology indications include APIs indicated for Angina, Dyslipidemia, Heart Rhythm, Myocardial Infarction, and Peripheral Blood flow.
Drug Strength and Dosing
The visualization on the below illustrates the formulation challenges for OSDF service and technology suppliers. The wide range of dosing categories and the resulting process and QA issues they represent presents opportunities as well facility and equipment issues.
SAMPLE VIEW

Table of Contents
- Oral Solid Dosage Forms for Cardiology
- Executive Summary
- Generic SDFs - Product Considerations
- Generic Solid Dosage Forms - Cardiology Indications
- Hypertension
- Cardiology Therapeutic Classes: All Classes excluding Hypertension
- Leading Suppliers of OSDF Drugs Indicated for Hypertension
- Drug Class - Market Presence by Supplier
- Drug Class - ACE Inhibitors (Abbvie o Graviti Pharms)
- Drug Class - Ace Inhibitors (Graviti Pharms to Zydus Pharma USA)
- Drug Class - Adrenoceptor Blocking Agent (Able to Mylan)
- Drug Class - Adrenoceptor Blocking Agent (Mylan to Zydus Pharms)
- Drug Class - Angiotensin II Receptor (Ajanta Pharma Ltd to Zydus Pharms)
- Drug Class - Calcium Channel Blocker (Accord Healthcare to Mutual Pharm)
- Drug Class - Calcium Channel Blocker (Mylan to Zydus Pharms)
- Drug Class - Diuretic (Actavis to Monarch Pharms)
- Drug Class - Diuretic (Actavis to Monarch Pharms)
- Drug Class - Vasodilator (Actavis to Woodward)
- Suppliers of OSDFs Indicated for Cardiology
- Accord Healthcare
- Actavis
- Alembic
- Amneal Pharms
- ANI Pharms
- Apotex
- Aurobindo
- Dr. Reddy's
- Glenmark
- Hikma
- Lupin
- Mylan
- Mylan (Continued)
- Sandoz
- Sun Pharma
- Teva
- Torrent Pharms
- Watson Labs
- Zydus Pharms USA
- OSDF - Dosage Maps











