 |
市场调查报告书
商品编码
1412615
癌症免疫学临床试验市场:依设计、阶段、适应症划分 - 2024-2030 年全球预测Immuno-oncology Clinical Trials Market by Design (Expanded Access Trials, Interventional Trials, Observational Trials), Phase (Phase I, Phase II, Phase III), Indication - Global Forecast 2024-2030 |
||||||
※ 本网页内容可能与最新版本有所差异。详细情况请与我们联繫。
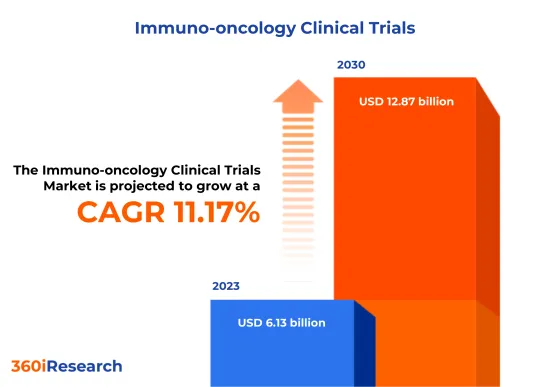
预计2023年癌症免疫临床试验市场规模为61.3亿美元,2024年达65.8亿美元,预计2030年将达到128.7亿美元,复合年增长率为11.17%。
癌症免疫学临床试验的全球市场
| 主要市场统计 | |
|---|---|
| 基准年[2023] | 61.3亿美元 |
| 预测年份 [2024] | 65.8亿美元 |
| 预测年份 [2030] | 128.7亿美元 |
| 复合年增长率(%) | 11.17% |
FPNV定位矩阵
FPNV定位矩阵对于评估免疫肿瘤学临床试验市场至关重要。我们检视与业务策略和产品满意度相关的关键指标,以对供应商进行全面评估。这种深入的分析使用户能够根据自己的要求做出明智的决策。根据评估,供应商被分为四个成功程度不同的像限:前沿(F)、探路者(P)、利基(N)和重要(V)。
市场占有率分析
市场占有率分析是一种综合工具,可以对免疫肿瘤临床试验市场供应商的现状进行深入而深入的研究。全面比较和分析供应商在整体收益、基本客群和其他关键指标方面的贡献,以便更好地了解公司的绩效及其在争夺市场占有率时面临的挑战。此外,该分析还提供了对该行业竞争特征的宝贵考察,包括在研究基准年观察到的累积、分散主导地位和合併特征等因素。这种详细程度的提高使供应商能够做出更明智的决策并制定有效的策略,从而在市场上获得竞争优势。
该报告对以下几个方面提供了宝贵的见解:
1-市场渗透率:提供有关主要企业所服务的市场的全面资讯。
2-市场开拓:我们深入研究利润丰厚的新兴市场,并分析它们在成熟细分市场中的渗透率。
3- 市场多元化:提供有关新产品发布、开拓地区、最新发展和投资的详细资讯。
4-竞争力评估与资讯:对主要企业的市场占有率、策略、产品、认证、监管状况、专利状况、製造能力等进行全面评估。
5- 产品开发与创新:提供对未来技术、研发活动和突破性产品开发的见解。
本报告解决了以下关键问题:
1-癌症免疫学临床试验市场的市场规模与预测是多少?
2-肿瘤免疫学临床试验市场预测期间需要考虑投资的产品、细分市场、应用和领域有哪些?
3-癌症免疫学临床试验市场的技术趋势和法律规范是什么?
4-癌症免疫学临床试验市场主要厂商的市场占有率是多少?
5-进入癌症免疫学临床试验市场的合适型态和策略手段是什么?
目录
第一章 前言
第二章调查方法
第三章执行摘要
第四章市场概况
第五章市场洞察
- 市场动态
- 促进因素
- 癌症发生率上升和标靶治疗的增加
- 伴同性诊断在药物开发中日益重要
- 个人化医疗的需求正在迅速成长
- 抑制因素
- 需要大量资金投入,成本效益不高
- 机会
- 癌症免疫检测的进展
- 增加政府对癌症研究和开发的财政支持
- 任务
- 高度复杂的生产製造工艺
- 促进因素
- 市场区隔分析
- 市场趋势分析
- 高通膨的累积效应
- 波特五力分析
- 价值炼和关键路径分析
- 法律规范
第六章癌症免疫学临床试验市场:设计
- 扩展访问考试
- 干预试验
- 观察测试
第七章癌症免疫学临床试验市场:依阶段
- 第一阶段
- 第二阶段
- 第三阶段
- 第四阶段
第八章癌症免疫学临床试验市场:依适应症分类
- 血癌
- 固态肿瘤
第九章美洲癌症免疫学临床试验市场
- 阿根廷
- 巴西
- 加拿大
- 墨西哥
- 美国
第十章亚太癌症免疫学临床试验市场
- 澳洲
- 中国
- 印度
- 印尼
- 日本
- 马来西亚
- 菲律宾
- 新加坡
- 韩国
- 台湾
- 泰国
- 越南
第十一章 欧洲、中东、非洲癌症免疫学临床试验市场
- 丹麦
- 埃及
- 芬兰
- 法国
- 德国
- 以色列
- 义大利
- 荷兰
- 奈及利亚
- 挪威
- 波兰
- 卡达
- 俄罗斯
- 沙乌地阿拉伯
- 南非
- 西班牙
- 瑞典
- 瑞士
- 土耳其
- 阿拉伯聯合大公国
- 英国
第十二章竞争形势
- FPNV定位矩阵
- 市场占有率分析:主要企业
- 主要企业竞争情境分析
第13章竞争产品组合
- 主要公司简介
- AstraZeneca PLC
- BioNTech SE
- Bristol Myers Squibb Company
- Exscientia PLC
- F. Hoffmann-La Roche Ltd.
- ICON PLC
- IO Biotech ApS
- IQVIA Inc.
- Laboratory Corporation of America Holdings
- Medpace, Inc.
- Merck KGaA
- Novartis AG
- Pfizer Inc.
- Rubius Therapeutics, Inc.
- Syneos Health, Inc.
- 主要产品系列
第十四章附录
- 讨论指南
- 关于许可证和定价
[197 Pages Report] The Immuno-oncology Clinical Trials Market size was estimated at USD 6.13 billion in 2023 and expected to reach USD 6.58 billion in 2024, at a CAGR 11.17% to reach USD 12.87 billion by 2030.
Global Immuno-oncology Clinical Trials Market
| KEY MARKET STATISTICS | |
|---|---|
| Base Year [2023] | USD 6.13 billion |
| Estimated Year [2024] | USD 6.58 billion |
| Forecast Year [2030] | USD 12.87 billion |
| CAGR (%) | 11.17% |

FPNV Positioning Matrix
The FPNV Positioning Matrix is pivotal in evaluating the Immuno-oncology Clinical Trials Market. It offers a comprehensive assessment of vendors, examining key metrics related to Business Strategy and Product Satisfaction. This in-depth analysis empowers users to make well-informed decisions aligned with their requirements. Based on the evaluation, the vendors are then categorized into four distinct quadrants representing varying levels of success: Forefront (F), Pathfinder (P), Niche (N), or Vital (V).
Market Share Analysis
The Market Share Analysis is a comprehensive tool that provides an insightful and in-depth examination of the current state of vendors in the Immuno-oncology Clinical Trials Market. By meticulously comparing and analyzing vendor contributions in terms of overall revenue, customer base, and other key metrics, we can offer companies a greater understanding of their performance and the challenges they face when competing for market share. Additionally, this analysis provides valuable insights into the competitive nature of the sector, including factors such as accumulation, fragmentation dominance, and amalgamation traits observed over the base year period studied. With this expanded level of detail, vendors can make more informed decisions and devise effective strategies to gain a competitive edge in the market.
Key Company Profiles
The report delves into recent significant developments in the Immuno-oncology Clinical Trials Market, highlighting leading vendors and their innovative profiles. These include AstraZeneca PLC, BioNTech SE, Bristol Myers Squibb Company, Exscientia PLC, F. Hoffmann-La Roche Ltd., ICON PLC, IO Biotech ApS, IQVIA Inc., Laboratory Corporation of America Holdings, Medpace, Inc., Merck KGaA, Novartis AG, Pfizer Inc., Rubius Therapeutics, Inc., and Syneos Health, Inc..
Market Segmentation & Coverage
This research report categorizes the Immuno-oncology Clinical Trials Market to forecast the revenues and analyze trends in each of the following sub-markets:
- Design
- Expanded Access Trials
- Interventional Trials
- Observational Trials
- Phase
- Phase I
- Phase II
- Phase III
- Phase IV
- Indication
- Hematological Cancer
- Solid Tumors
- Region
- Americas
- Argentina
- Brazil
- Canada
- Mexico
- United States
- California
- Florida
- Illinois
- New York
- Ohio
- Pennsylvania
- Texas
- Asia-Pacific
- Australia
- China
- India
- Indonesia
- Japan
- Malaysia
- Philippines
- Singapore
- South Korea
- Taiwan
- Thailand
- Vietnam
- Europe, Middle East & Africa
- Denmark
- Egypt
- Finland
- France
- Germany
- Israel
- Italy
- Netherlands
- Nigeria
- Norway
- Poland
- Qatar
- Russia
- Saudi Arabia
- South Africa
- Spain
- Sweden
- Switzerland
- Turkey
- United Arab Emirates
- United Kingdom
- Americas
The report offers valuable insights on the following aspects:
1. Market Penetration: It presents comprehensive information on the market provided by key players.
2. Market Development: It delves deep into lucrative emerging markets and analyzes the penetration across mature market segments.
3. Market Diversification: It provides detailed information on new product launches, untapped geographic regions, recent developments, and investments.
4. Competitive Assessment & Intelligence: It conducts an exhaustive assessment of market shares, strategies, products, certifications, regulatory approvals, patent landscape, and manufacturing capabilities of the leading players.
5. Product Development & Innovation: It offers intelligent insights on future technologies, R&D activities, and breakthrough product developments.
The report addresses key questions such as:
1. What is the market size and forecast of the Immuno-oncology Clinical Trials Market?
2. Which products, segments, applications, and areas should one consider investing in over the forecast period in the Immuno-oncology Clinical Trials Market?
3. What are the technology trends and regulatory frameworks in the Immuno-oncology Clinical Trials Market?
4. What is the market share of the leading vendors in the Immuno-oncology Clinical Trials Market?
5. Which modes and strategic moves are suitable for entering the Immuno-oncology Clinical Trials Market?
Table of Contents
1. Preface
- 1.1. Objectives of the Study
- 1.2. Market Segmentation & Coverage
- 1.3. Years Considered for the Study
- 1.4. Currency & Pricing
- 1.5. Language
- 1.6. Limitations
- 1.7. Assumptions
- 1.8. Stakeholders
2. Research Methodology
- 2.1. Define: Research Objective
- 2.2. Determine: Research Design
- 2.3. Prepare: Research Instrument
- 2.4. Collect: Data Source
- 2.5. Analyze: Data Interpretation
- 2.6. Formulate: Data Verification
- 2.7. Publish: Research Report
- 2.8. Repeat: Report Update
3. Executive Summary
4. Market Overview
- 4.1. Introduction
- 4.2. Immuno-oncology Clinical Trials Market, by Region
5. Market Insights
- 5.1. Market Dynamics
- 5.1.1. Drivers
- 5.1.1.1. Rising incidence of cancer and the growing adoption of targeted therapy
- 5.1.1.2. Growing significance of companion diagnostics in drug development
- 5.1.1.3. Rapidly increasing demand for personalized medicine
- 5.1.2. Restraints
- 5.1.2.1. Requirement of high capital investments and low cost-benefit ratio
- 5.1.3. Opportunities
- 5.1.3.1. Advancements in immuno-oncology testing
- 5.1.3.2. Increasing financial support from the government for cancer R&D
- 5.1.4. Challenges
- 5.1.4.1. Huge complexity in the production and manufacturing processes
- 5.1.1. Drivers
- 5.2. Market Segmentation Analysis
- 5.3. Market Trend Analysis
- 5.4. Cumulative Impact of High Inflation
- 5.5. Porter's Five Forces Analysis
- 5.5.1. Threat of New Entrants
- 5.5.2. Threat of Substitutes
- 5.5.3. Bargaining Power of Customers
- 5.5.4. Bargaining Power of Suppliers
- 5.5.5. Industry Rivalry
- 5.6. Value Chain & Critical Path Analysis
- 5.7. Regulatory Framework
6. Immuno-oncology Clinical Trials Market, by Design
- 6.1. Introduction
- 6.2. Expanded Access Trials
- 6.3. Interventional Trials
- 6.4. Observational Trials
7. Immuno-oncology Clinical Trials Market, by Phase
- 7.1. Introduction
- 7.2. Phase I
- 7.3. Phase II
- 7.4. Phase III
- 7.5. Phase IV
8. Immuno-oncology Clinical Trials Market, by Indication
- 8.1. Introduction
- 8.2. Hematological Cancer
- 8.3. Solid Tumors
9. Americas Immuno-oncology Clinical Trials Market
- 9.1. Introduction
- 9.2. Argentina
- 9.3. Brazil
- 9.4. Canada
- 9.5. Mexico
- 9.6. United States
10. Asia-Pacific Immuno-oncology Clinical Trials Market
- 10.1. Introduction
- 10.2. Australia
- 10.3. China
- 10.4. India
- 10.5. Indonesia
- 10.6. Japan
- 10.7. Malaysia
- 10.8. Philippines
- 10.9. Singapore
- 10.10. South Korea
- 10.11. Taiwan
- 10.12. Thailand
- 10.13. Vietnam
11. Europe, Middle East & Africa Immuno-oncology Clinical Trials Market
- 11.1. Introduction
- 11.2. Denmark
- 11.3. Egypt
- 11.4. Finland
- 11.5. France
- 11.6. Germany
- 11.7. Israel
- 11.8. Italy
- 11.9. Netherlands
- 11.10. Nigeria
- 11.11. Norway
- 11.12. Poland
- 11.13. Qatar
- 11.14. Russia
- 11.15. Saudi Arabia
- 11.16. South Africa
- 11.17. Spain
- 11.18. Sweden
- 11.19. Switzerland
- 11.20. Turkey
- 11.21. United Arab Emirates
- 11.22. United Kingdom
12. Competitive Landscape
- 12.1. FPNV Positioning Matrix
- 12.2. Market Share Analysis, By Key Player
- 12.3. Competitive Scenario Analysis, By Key Player
13. Competitive Portfolio
- 13.1. Key Company Profiles
- 13.1.1. AstraZeneca PLC
- 13.1.2. BioNTech SE
- 13.1.3. Bristol Myers Squibb Company
- 13.1.4. Exscientia PLC
- 13.1.5. F. Hoffmann-La Roche Ltd.
- 13.1.6. ICON PLC
- 13.1.7. IO Biotech ApS
- 13.1.8. IQVIA Inc.
- 13.1.9. Laboratory Corporation of America Holdings
- 13.1.10. Medpace, Inc.
- 13.1.11. Merck KGaA
- 13.1.12. Novartis AG
- 13.1.13. Pfizer Inc.
- 13.1.14. Rubius Therapeutics, Inc.
- 13.1.15. Syneos Health, Inc.
- 13.2. Key Product Portfolio
14. Appendix
- 14.1. Discussion Guide
- 14.2. License & Pricing
LIST OF FIGURES
- FIGURE 1. IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET RESEARCH PROCESS
- FIGURE 2. IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, 2023 VS 2030
- FIGURE 3. IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, 2018-2030 (USD MILLION)
- FIGURE 4. IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY REGION, 2023 VS 2030 (%)
- FIGURE 5. IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY REGION, 2023 VS 2024 VS 2030 (USD MILLION)
- FIGURE 6. IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET DYNAMICS
- FIGURE 7. IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY DESIGN, 2023 VS 2030 (%)
- FIGURE 8. IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY DESIGN, 2023 VS 2024 VS 2030 (USD MILLION)
- FIGURE 9. IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY PHASE, 2023 VS 2030 (%)
- FIGURE 10. IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY PHASE, 2023 VS 2024 VS 2030 (USD MILLION)
- FIGURE 11. IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY INDICATION, 2023 VS 2030 (%)
- FIGURE 12. IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY INDICATION, 2023 VS 2024 VS 2030 (USD MILLION)
- FIGURE 13. AMERICAS IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY COUNTRY, 2023 VS 2030 (%)
- FIGURE 14. AMERICAS IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY COUNTRY, 2023 VS 2024 VS 2030 (USD MILLION)
- FIGURE 15. UNITED STATES IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY STATE, 2023 VS 2030 (%)
- FIGURE 16. UNITED STATES IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY STATE, 2023 VS 2024 VS 2030 (USD MILLION)
- FIGURE 17. ASIA-PACIFIC IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY COUNTRY, 2023 VS 2030 (%)
- FIGURE 18. ASIA-PACIFIC IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY COUNTRY, 2023 VS 2024 VS 2030 (USD MILLION)
- FIGURE 19. EUROPE, MIDDLE EAST & AFRICA IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY COUNTRY, 2023 VS 2030 (%)
- FIGURE 20. EUROPE, MIDDLE EAST & AFRICA IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY COUNTRY, 2023 VS 2024 VS 2030 (USD MILLION)
- FIGURE 21. IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET, FPNV POSITIONING MATRIX, 2023
- FIGURE 22. IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SHARE, BY KEY PLAYER, 2023
LIST OF TABLES
- TABLE 1. IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SEGMENTATION & COVERAGE
- TABLE 2. UNITED STATES DOLLAR EXCHANGE RATE, 2018-2023
- TABLE 3. IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, 2018-2030 (USD MILLION)
- TABLE 4. GLOBAL IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY REGION, 2018-2030 (USD MILLION)
- TABLE 5. IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY DESIGN, 2018-2030 (USD MILLION)
- TABLE 6. IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY EXPANDED ACCESS TRIALS, BY REGION, 2018-2030 (USD MILLION)
- TABLE 7. IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY INTERVENTIONAL TRIALS, BY REGION, 2018-2030 (USD MILLION)
- TABLE 8. IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY OBSERVATIONAL TRIALS, BY REGION, 2018-2030 (USD MILLION)
- TABLE 9. IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY PHASE, 2018-2030 (USD MILLION)
- TABLE 10. IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY PHASE I, BY REGION, 2018-2030 (USD MILLION)
- TABLE 11. IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY PHASE II, BY REGION, 2018-2030 (USD MILLION)
- TABLE 12. IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY PHASE III, BY REGION, 2018-2030 (USD MILLION)
- TABLE 13. IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY PHASE IV, BY REGION, 2018-2030 (USD MILLION)
- TABLE 14. IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY INDICATION, 2018-2030 (USD MILLION)
- TABLE 15. IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY HEMATOLOGICAL CANCER, BY REGION, 2018-2030 (USD MILLION)
- TABLE 16. IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY SOLID TUMORS, BY REGION, 2018-2030 (USD MILLION)
- TABLE 17. AMERICAS IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY DESIGN, 2018-2030 (USD MILLION)
- TABLE 18. AMERICAS IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY PHASE, 2018-2030 (USD MILLION)
- TABLE 19. AMERICAS IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY INDICATION, 2018-2030 (USD MILLION)
- TABLE 20. AMERICAS IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY COUNTRY, 2018-2030 (USD MILLION)
- TABLE 21. ARGENTINA IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY DESIGN, 2018-2030 (USD MILLION)
- TABLE 22. ARGENTINA IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY PHASE, 2018-2030 (USD MILLION)
- TABLE 23. ARGENTINA IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY INDICATION, 2018-2030 (USD MILLION)
- TABLE 24. BRAZIL IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY DESIGN, 2018-2030 (USD MILLION)
- TABLE 25. BRAZIL IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY PHASE, 2018-2030 (USD MILLION)
- TABLE 26. BRAZIL IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY INDICATION, 2018-2030 (USD MILLION)
- TABLE 27. CANADA IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY DESIGN, 2018-2030 (USD MILLION)
- TABLE 28. CANADA IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY PHASE, 2018-2030 (USD MILLION)
- TABLE 29. CANADA IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY INDICATION, 2018-2030 (USD MILLION)
- TABLE 30. MEXICO IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY DESIGN, 2018-2030 (USD MILLION)
- TABLE 31. MEXICO IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY PHASE, 2018-2030 (USD MILLION)
- TABLE 32. MEXICO IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY INDICATION, 2018-2030 (USD MILLION)
- TABLE 33. UNITED STATES IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY DESIGN, 2018-2030 (USD MILLION)
- TABLE 34. UNITED STATES IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY PHASE, 2018-2030 (USD MILLION)
- TABLE 35. UNITED STATES IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY INDICATION, 2018-2030 (USD MILLION)
- TABLE 36. UNITED STATES IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY STATE, 2018-2030 (USD MILLION)
- TABLE 37. ASIA-PACIFIC IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY DESIGN, 2018-2030 (USD MILLION)
- TABLE 38. ASIA-PACIFIC IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY PHASE, 2018-2030 (USD MILLION)
- TABLE 39. ASIA-PACIFIC IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY INDICATION, 2018-2030 (USD MILLION)
- TABLE 40. ASIA-PACIFIC IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY COUNTRY, 2018-2030 (USD MILLION)
- TABLE 41. AUSTRALIA IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY DESIGN, 2018-2030 (USD MILLION)
- TABLE 42. AUSTRALIA IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY PHASE, 2018-2030 (USD MILLION)
- TABLE 43. AUSTRALIA IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY INDICATION, 2018-2030 (USD MILLION)
- TABLE 44. CHINA IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY DESIGN, 2018-2030 (USD MILLION)
- TABLE 45. CHINA IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY PHASE, 2018-2030 (USD MILLION)
- TABLE 46. CHINA IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY INDICATION, 2018-2030 (USD MILLION)
- TABLE 47. INDIA IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY DESIGN, 2018-2030 (USD MILLION)
- TABLE 48. INDIA IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY PHASE, 2018-2030 (USD MILLION)
- TABLE 49. INDIA IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY INDICATION, 2018-2030 (USD MILLION)
- TABLE 50. INDONESIA IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY DESIGN, 2018-2030 (USD MILLION)
- TABLE 51. INDONESIA IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY PHASE, 2018-2030 (USD MILLION)
- TABLE 52. INDONESIA IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY INDICATION, 2018-2030 (USD MILLION)
- TABLE 53. JAPAN IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY DESIGN, 2018-2030 (USD MILLION)
- TABLE 54. JAPAN IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY PHASE, 2018-2030 (USD MILLION)
- TABLE 55. JAPAN IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY INDICATION, 2018-2030 (USD MILLION)
- TABLE 56. MALAYSIA IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY DESIGN, 2018-2030 (USD MILLION)
- TABLE 57. MALAYSIA IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY PHASE, 2018-2030 (USD MILLION)
- TABLE 58. MALAYSIA IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY INDICATION, 2018-2030 (USD MILLION)
- TABLE 59. PHILIPPINES IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY DESIGN, 2018-2030 (USD MILLION)
- TABLE 60. PHILIPPINES IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY PHASE, 2018-2030 (USD MILLION)
- TABLE 61. PHILIPPINES IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY INDICATION, 2018-2030 (USD MILLION)
- TABLE 62. SINGAPORE IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY DESIGN, 2018-2030 (USD MILLION)
- TABLE 63. SINGAPORE IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY PHASE, 2018-2030 (USD MILLION)
- TABLE 64. SINGAPORE IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY INDICATION, 2018-2030 (USD MILLION)
- TABLE 65. SOUTH KOREA IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY DESIGN, 2018-2030 (USD MILLION)
- TABLE 66. SOUTH KOREA IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY PHASE, 2018-2030 (USD MILLION)
- TABLE 67. SOUTH KOREA IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY INDICATION, 2018-2030 (USD MILLION)
- TABLE 68. TAIWAN IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY DESIGN, 2018-2030 (USD MILLION)
- TABLE 69. TAIWAN IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY PHASE, 2018-2030 (USD MILLION)
- TABLE 70. TAIWAN IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY INDICATION, 2018-2030 (USD MILLION)
- TABLE 71. THAILAND IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY DESIGN, 2018-2030 (USD MILLION)
- TABLE 72. THAILAND IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY PHASE, 2018-2030 (USD MILLION)
- TABLE 73. THAILAND IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY INDICATION, 2018-2030 (USD MILLION)
- TABLE 74. VIETNAM IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY DESIGN, 2018-2030 (USD MILLION)
- TABLE 75. VIETNAM IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY PHASE, 2018-2030 (USD MILLION)
- TABLE 76. VIETNAM IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY INDICATION, 2018-2030 (USD MILLION)
- TABLE 77. EUROPE, MIDDLE EAST & AFRICA IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY DESIGN, 2018-2030 (USD MILLION)
- TABLE 78. EUROPE, MIDDLE EAST & AFRICA IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY PHASE, 2018-2030 (USD MILLION)
- TABLE 79. EUROPE, MIDDLE EAST & AFRICA IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY INDICATION, 2018-2030 (USD MILLION)
- TABLE 80. EUROPE, MIDDLE EAST & AFRICA IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY COUNTRY, 2018-2030 (USD MILLION)
- TABLE 81. DENMARK IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY DESIGN, 2018-2030 (USD MILLION)
- TABLE 82. DENMARK IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY PHASE, 2018-2030 (USD MILLION)
- TABLE 83. DENMARK IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY INDICATION, 2018-2030 (USD MILLION)
- TABLE 84. EGYPT IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY DESIGN, 2018-2030 (USD MILLION)
- TABLE 85. EGYPT IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY PHASE, 2018-2030 (USD MILLION)
- TABLE 86. EGYPT IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY INDICATION, 2018-2030 (USD MILLION)
- TABLE 87. FINLAND IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY DESIGN, 2018-2030 (USD MILLION)
- TABLE 88. FINLAND IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY PHASE, 2018-2030 (USD MILLION)
- TABLE 89. FINLAND IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY INDICATION, 2018-2030 (USD MILLION)
- TABLE 90. FRANCE IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY DESIGN, 2018-2030 (USD MILLION)
- TABLE 91. FRANCE IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY PHASE, 2018-2030 (USD MILLION)
- TABLE 92. FRANCE IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY INDICATION, 2018-2030 (USD MILLION)
- TABLE 93. GERMANY IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY DESIGN, 2018-2030 (USD MILLION)
- TABLE 94. GERMANY IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY PHASE, 2018-2030 (USD MILLION)
- TABLE 95. GERMANY IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY INDICATION, 2018-2030 (USD MILLION)
- TABLE 96. ISRAEL IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY DESIGN, 2018-2030 (USD MILLION)
- TABLE 97. ISRAEL IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY PHASE, 2018-2030 (USD MILLION)
- TABLE 98. ISRAEL IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY INDICATION, 2018-2030 (USD MILLION)
- TABLE 99. ITALY IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY DESIGN, 2018-2030 (USD MILLION)
- TABLE 100. ITALY IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY PHASE, 2018-2030 (USD MILLION)
- TABLE 101. ITALY IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY INDICATION, 2018-2030 (USD MILLION)
- TABLE 102. NETHERLANDS IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY DESIGN, 2018-2030 (USD MILLION)
- TABLE 103. NETHERLANDS IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY PHASE, 2018-2030 (USD MILLION)
- TABLE 104. NETHERLANDS IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY INDICATION, 2018-2030 (USD MILLION)
- TABLE 105. NIGERIA IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY DESIGN, 2018-2030 (USD MILLION)
- TABLE 106. NIGERIA IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY PHASE, 2018-2030 (USD MILLION)
- TABLE 107. NIGERIA IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY INDICATION, 2018-2030 (USD MILLION)
- TABLE 108. NORWAY IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY DESIGN, 2018-2030 (USD MILLION)
- TABLE 109. NORWAY IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY PHASE, 2018-2030 (USD MILLION)
- TABLE 110. NORWAY IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY INDICATION, 2018-2030 (USD MILLION)
- TABLE 111. POLAND IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY DESIGN, 2018-2030 (USD MILLION)
- TABLE 112. POLAND IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY PHASE, 2018-2030 (USD MILLION)
- TABLE 113. POLAND IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY INDICATION, 2018-2030 (USD MILLION)
- TABLE 114. QATAR IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY DESIGN, 2018-2030 (USD MILLION)
- TABLE 115. QATAR IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY PHASE, 2018-2030 (USD MILLION)
- TABLE 116. QATAR IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY INDICATION, 2018-2030 (USD MILLION)
- TABLE 117. RUSSIA IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY DESIGN, 2018-2030 (USD MILLION)
- TABLE 118. RUSSIA IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY PHASE, 2018-2030 (USD MILLION)
- TABLE 119. RUSSIA IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY INDICATION, 2018-2030 (USD MILLION)
- TABLE 120. SAUDI ARABIA IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY DESIGN, 2018-2030 (USD MILLION)
- TABLE 121. SAUDI ARABIA IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY PHASE, 2018-2030 (USD MILLION)
- TABLE 122. SAUDI ARABIA IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY INDICATION, 2018-2030 (USD MILLION)
- TABLE 123. SOUTH AFRICA IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY DESIGN, 2018-2030 (USD MILLION)
- TABLE 124. SOUTH AFRICA IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY PHASE, 2018-2030 (USD MILLION)
- TABLE 125. SOUTH AFRICA IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY INDICATION, 2018-2030 (USD MILLION)
- TABLE 126. SPAIN IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY DESIGN, 2018-2030 (USD MILLION)
- TABLE 127. SPAIN IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY PHASE, 2018-2030 (USD MILLION)
- TABLE 128. SPAIN IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY INDICATION, 2018-2030 (USD MILLION)
- TABLE 129. SWEDEN IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY DESIGN, 2018-2030 (USD MILLION)
- TABLE 130. SWEDEN IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY PHASE, 2018-2030 (USD MILLION)
- TABLE 131. SWEDEN IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY INDICATION, 2018-2030 (USD MILLION)
- TABLE 132. SWITZERLAND IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY DESIGN, 2018-2030 (USD MILLION)
- TABLE 133. SWITZERLAND IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY PHASE, 2018-2030 (USD MILLION)
- TABLE 134. SWITZERLAND IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY INDICATION, 2018-2030 (USD MILLION)
- TABLE 135. TURKEY IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY DESIGN, 2018-2030 (USD MILLION)
- TABLE 136. TURKEY IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY PHASE, 2018-2030 (USD MILLION)
- TABLE 137. TURKEY IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY INDICATION, 2018-2030 (USD MILLION)
- TABLE 138. UNITED ARAB EMIRATES IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY DESIGN, 2018-2030 (USD MILLION)
- TABLE 139. UNITED ARAB EMIRATES IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY PHASE, 2018-2030 (USD MILLION)
- TABLE 140. UNITED ARAB EMIRATES IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY INDICATION, 2018-2030 (USD MILLION)
- TABLE 141. UNITED KINGDOM IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY DESIGN, 2018-2030 (USD MILLION)
- TABLE 142. UNITED KINGDOM IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY PHASE, 2018-2030 (USD MILLION)
- TABLE 143. UNITED KINGDOM IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SIZE, BY INDICATION, 2018-2030 (USD MILLION)
- TABLE 144. IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET, FPNV POSITIONING MATRIX, 2023
- TABLE 145. IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET SHARE, BY KEY PLAYER, 2023
- TABLE 146. IMMUNO-ONCOLOGY CLINICAL TRIALS MARKET LICENSE & PRICING













