 |
市场调查报告书
商品编码
1089231
Dravet 综合征 (DS) 市场:初步调查(竞争信息、市场分析和 2032 年预测)Dravet Syndrome (DS) | Primary Research (KOL's Insight) | Competitive Intelligence | Market Analytics & Forecast 2032 |
||||||
本报告调查了全球 Dravet 综合征 (DS) 市场,并提供了市场概况、2032 年预测、当前治疗和未满足的需求、国家趋势、市场驱动因素和限制等。
目录
执行摘要
- 主要调查结果
Dravet 综合征 (DS) 疾病的背景
- Dravet 综合征 (DS) 的定义
- 症状和原因
- SCN1A基因
- 诊断
- 鑑别诊断
到 2032 年的流行病学和预测
- 主要调查结果
- 方法和数据源
Dravet 综合征(DS)流行病学和模型参数的主要来源
- 巴西
- 墨西哥
当前的治疗和医疗实践
- 治疗和医疗实践
- Dravet 综合征 (DS) 癫痫发作的治疗
- 自 2017 年以来获得美国 FDA 批准的治疗 Dravet 综合征的药物
- 大麻二酚治疗 Dravet 综合征 (DS)
未满足的需求
治疗章节
新疗法
Dravet 综合症 (DS) - 价格和赎回
未来治疗范式
- Situation Syndrome (DS) 竞争对手情况和预期批准
- 未来的治疗算法和竞争对手定位
- 新疗法的重要数据摘要
当前和新疗法的年度费用
市场展望
到 2032 年的国家市场预测
各国市场预测
- 巴西
- 墨西哥
市场促进因素和限制
报告调查方法
附录
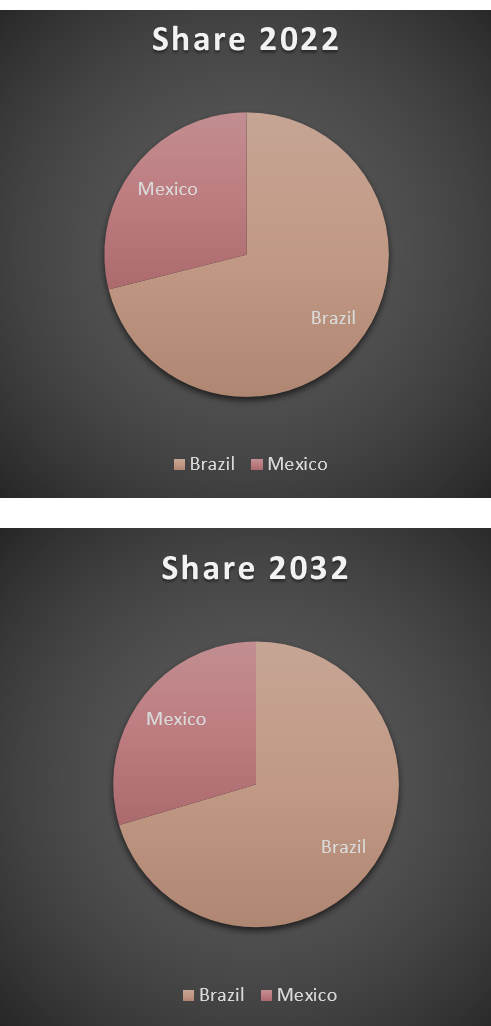
Growth of the incidence DS population will contribute to increasing sales in the DS therapy market over our forecast period. An estimated annual growth rate of ~1% in the diagnosed incident DS population across the major pharmaceutical markets will contribute to increasing DS therapy sales. In 2018, the drug-treated cases of Dravet Syndrome (DS) in Brazil & Mexico were 8,392 cases. These cases are expected to increase to 11,335 cases in 2032 owing to changes in population demographics and increasing cases of DS.
The continuous involvement of supportive treatments including antiepileptic therapies for the management of DS patients will contribute to the overall growth of the DS therapy market. We forecast that total sales of the total care market will reach $276 Million by 2032.
Dravet syndrome is a severe form of epilepsy characterized by frequent, prolonged seizures often triggered by high body temperature (hyperthermia), developmental delay, speech impairment, ataxia, hypotonia, sleep disturbances, and other health problems. DS is considered an epileptic encephalopathy due to seizures. In addition, it is considered a "channelopathy" because the effects of the mutation on the sodium channel appear to contribute to the disorder independently of the seizures.
Dravet Syndrome - DS Epidemiology
We estimate the total number of Dravet Syndrome (DS) prevalent cases in the Brazil and Mexico of 1:20,000 or 0.005%. There is a significant amount of shortage of large-scale epidemiological reports. The rarity of the disease further compounds the problem at hand as there exists a lack of firmly established and specific diagnostic criteria and some of the studies may have chances of biasness depending on the geographical area studied. There also exists methodological challenges for researchers due to measurements involving a small sample population.

According to the KOLs View, approximately 50% of the cases of Dravet Syndrome (DS) are diagnosed. Patients with Dravet syndrome may be misdiagnosed with myoclonic atonic epilepsy, Lennox-Gastaut syndrome, myoclonic epilepsy of infancy, genetic epilepsy with febrile seizures plus, atypical febrile seizures, and mitochondrial disorders. Additionally, some children may be diagnosed with focal epilepsy.
Dravet Syndrome -DS Market Forecast
The Dravet Syndrome (DS) therapy market is expected to experience high growth throughout our study period, increasing from $XX billion in 2018 to $XX billion in 2032, representing ~1% annual growth. Primary drivers of this growth will be uptake of the novel upcoming treatment like Soticlestat (Takeda/Ovid Therapeutics), Lorcaserin (Eisai/Arena Pharmaceuticals), STK 001 (Stoke Therapeutics, Inc), EPX 100 (Epygenix Therapeutics), ETX101 (Encoded Therapeutics).
Most interviewed experts believe that patients will benefit from the upcoming therapies based on targeting CH24H inhibitors, selective serotonin 5-HT2c receptor, NAV1.1 Voltage-gated Sodium Channel Modulators and Specific GABAA Receptor have the potential to increase the therapeutic options for Dravet Syndrome (DS) patients. Expected launch of late-stage product such as Soticlestat, Lorcaserin and STK 001 are likely to change the current treatment paradigm for Dravet Syndrome (DS) and these approved therapies will be more preferrable in the future.

Report Highlights
- Dravet Syndrome-DS Current Market Trends
- Dravet Syndrome-DS Current & Forecasted Cases across Brazil & Mexico
- Dravet Syndrome-DS Market Opportunities And Sales Potential for Agents
- Dravet Syndrome-DS Patient-based Market Forecast to 2032
- Dravet Syndrome-DS Untapped Business Opportunities
- Dravet Syndrome-DS Product Positioning Vis-a-vis Competitors' Products
- Dravet Syndrome-DS KOLs Insight
Table of Contents
Executive Summary
- Key Findings
Dravet Syndrome (DS) Disease Background
- Dravet Syndrome (DS) Definition
- Symptoms & Causes
- SCN1A Gene
- Diagnosis
- Differential Diagnosis
Epidemiology Estimated and Forecast to 2032
- Key Findings
- Methods and data Sources
- Country Specific Prevalent cases of Dravet Syndrome (DS) (Brazil and Mexico)
- Country Specific Diagnosed and Drug Treated Cases of Dravet Syndrome (DS)
Key Sources for Dravet Syndrome (DS) Epidemiology and Model Parameters
- Brazil
- Brazil Prevalent cases of Dravet Syndrome (DS)
- Brazil Diagnosed and Drug Treated Cases of Dravet Syndrome (DS)
- Mexico
- Mexico Prevalent cases of Dravet Syndrome (DS)
- Mexico Diagnosed and Drug Treated Cases of Dravet Syndrome (DS)
Current Therapies and Medical Practice
- Treatments & Medical Practices
- Treatment of Seizures in Dravet Syndrome (DS)
- Approved Therapies for Dravet Syndrome by U.S. FDA since 2017
- Cannabidiol in the treatment of Dravet Syndrome (DS)
Unmet Needs
Therapies Chapters
- Fenfluramine (Zogenix)
- Epidiolex (Jazz Pharmaceuticals plc/GW Pharmaceuticals)
- Stiripentol (Biocodex)
Emerging Therapies
- Pipeline Overview
- Therapeutic Developments Pipeline for Dravet Syndrome (DS)
- Product Analysis
- Soticlestat (Takeda/Ovid Therapeutics)
- Product Profile
- Clinical Development
- Sales & Market Opportunity by 2032
- Lorcaserin (Eisai/Arena Pharmaceuticals)
- Product Profile
- Clinical Development
- Sales & Market Opportunity by 2032
- STK 001 (Stoke Therapeutics)
- Product Profile
- Clinical Development
- Sales & Market Opportunity by 2032
- EPX 100 (Epygenix Therapeutics)
- Product Profile
- Clinical Development
- Sales & Market Opportunity by 2032
- EPX 200 (Epygenix Therapeutics)
- Product Profile
- Sales & Market Opportunity by 2032
- EPX 300 (Epygenix Therapeutics)
- Product Profile
- Sales & Market Opportunity by 2032
- ETX101 (Encoded Therapeutics)
- Product Profile
- Sales & Market Opportunity by 2032
- Huperzine A (Supernus Pharmaceuticals/Biscayne Neurotherapeutics)
- Product Profile
- LP352 (Longboard Pharmaceuticals/Arena Pharmaceuticals)
- Product Profile
- NT-102 (Neuroene Therapeutics)
- Product Profile
- NCT10004 (Engrail Therapeutics/NeuroCycle Therapeutics)
- Product Profile
- BMB-101 (Bright Minds Biosciences)
- Product Profile
- OPK88001 (CAMP4 Therapeutics/OPKO Health)
- Product Profile
- Soticlestat (Takeda/Ovid Therapeutics)
- Product Analysis
Dravet Syndrome (DS)- Pricing & Reimbursement
Future Treatment Paradigm
- Dravet Syndrome (DS) Competitor Landscape and Approvals Anticipated
- Future Treatment Algorithms and Competitor Positioning
- Key Data Summary for Emerging Treatment
Annual Cost of Current & Emerging Therapies
Market Outlook
- Key Findings
Country Specific Market Forecast to 2032
- Total Market for Dravet Syndrome (DS) 2018-2032 (USD Million)
- Total Market for Dravet Syndrome (DS) by Therapies 2018-2032 (USD Million)
Market Forecast by Country
- Brazil
- Brazil Market for Dravet Syndrome (DS) 2018-2032 (USD Million)
- Brazil Market for Dravet Syndrome (DS) by Therapies 2018-2032 (USD Million)
- Mexico
- Mexico Market for Dravet Syndrome (DS) 2018-2032 (USD Million)
- Mexico Market for Dravet Syndrome (DS) by Therapies 2018-2032 (USD Million)
Market Drivers and Constraints
- What Factors Are Driving the Market for Dravet Syndrome (DS)?
- What Factors Are Constraining the Market for Dravet Syndrome (DS)?






