 |
市场调查报告书
商品编码
1237835
高通量过程开发市场——增长、趋势和预测 (2023-2028)High Throughput Process Development Market - Growth, Trends, And Forecasts (2023 - 2028) |
||||||
※ 本网页内容可能与最新版本有所差异。详细情况请与我们联繫。
在预测期内,高通量工艺开发市场预计将以 14.4% 的复合年增长率增长。
由于全球研发活动中断,COVID-19 大流行对其前身的高通量工艺开发 (HTPD) 市场产生了重大影响。 随着大流行的持续恶化,我们加快了针对 SARS-CoV-2 病毒的疫苗和药物开发进程。 根据 HTPD 精准医学综合解决方案提供商 BGI Genomics 2022 年 11 月的更新,随着大流行的爆发,贝尔格莱德大学分子遗传工程研究所 (IMGGE) 立即扩大了其在分子诊断方面的专业知识。报价。 BGI与 IMGGE 的合作始于两个永久性火眼实验室的建设。 在宣布大流行大约 40 天后,第一个实验室设立在塞尔维亚大学临床中心的场地上。 在贝尔格莱德建立了两个传染病分子诊断国家实验室之一。 到 2020 年 7 月,战略性地位于塞尔维亚城市尼什的第二个国家实验室开始运作,为该地区提供服务。 这些新兴市场的发展为大流行期间的市场增长做出了贡献。
即使在大流行后阶段,市场也有望实现显着增长。 例如,根据 Frontiers in Medical Technology 于 2022 年 9 月发表的一篇论文,自动化微流体技术可以提高 COVID-19 诊断测试的可及性,并加快高通量检测技术的速度,尤其是对于 POC 测试。有相当大的保证 此外,使用为其他病原体製造的自动化微流体平台可以轻鬆进行 COVID-19 诊断。 因此,随着全球预计会出现新型 SARS-CoV-2 毒株,市场有望在未来几年获得牵引力。
此外,与 HTPD 相关的好处,例如确定新药目标的需求不断增长、药物发现和开发技术的进步、药物研发成本上升、成本降低和流程效率,可能会在未来几年市场发展的主要因素。
高通量筛选允许在相同条件下同时测试大量样本。 此外,通过使用机器人功能,可以同时检查数百或数千个样品。 对于大样本量,高通量工艺开发速度更快且成本更低,使其成为筛选新药物靶标以进行药物开发的便捷选择。 高通量开髮带来的这些好处预计将很快推动药物发现过程中的市场增长,在该过程中需要筛选多种候选药物。
此外,高通量测序技术将在 2020 年解码 100 万个人类基因组,从而彻底改变遗传学和基因组学。 因此,製药行业可以获得需要进一步研究的广泛治疗靶点,从而推动了对高通量工艺开发的需求。
同样,製药行业的研发进展也有望促进市场增长。 针对中枢神经系统 (CNS) 的药物历来在阿尔茨海默病等神经退行性疾□□病的临床试验中显示出高失败率。 此外,体内筛选耗时且成本高昂的负担仍然是临床前研究的重要组成部分。 近年来,高通量技术使这些测试能够满足当前药物开发步伐的需求。 例如,2021 年 6 月,法国阿图瓦大学的科学家将获得专利的 BBB 体外模型小型化,以实现自动化高通量化合物筛选。 我们使用机器人细胞接种、化验测试和成像来自动化以前使用 12 孔格式手动完成的模型。 自动化提高了准确性并减少了研究人员运行分析所需的时间。 准确性大大提高,减少了研究人员运行检测的时间。 最终,它使得在药物发现的早期阶段筛选更多化合物成为可能。
此外,新产品的发布也促进了市场的增长。 例如,2021 年 4 月,Thermo Fisher Scientific 的高通量集成 COVID-19 测试套件获得了 USFDA 的批准。 同样,在 2022 年 11 月,Roche的 COBAS MPXV 高通量测试获得了 FDA 的 EUA,用于检测 mpox。 患者可以通过这种高通量解决方案快速获得预期结果,尽快接受适当的治疗,而无需进行不必要的额外检测或隔离。 2021 年 12 月,Becton, Dickinson, and Company 的 COR 系统得到增强,增加了用于传染病高通量分子检测的新 MX 仪器。
因此,由于上述因素,预计所研究的市场在预测期内将出现显着增长。 然而,高技术成本和薄弱的基础设施可能会阻碍市场增长。
高通量工艺开发市场趋势
生物製药和生物技术公司有望显着增长
在最终用户方面,预计生物製药和生物技术公司将在分析期间实现显着增长。 这主要是由于公司研发活动和支出的增加以及为药物发现确定新药物靶点的需求不断增长。
此外,推动该细分市场增长的主要因素是这些公司大量采用高通量工艺开发、引入更新和更先进的平台、药物开发领域的技术进步以及主要参与者在市场上。它是由战略发展的增长 例如,2022 年 11 月,高通量单细胞 DNA 和多组学分析领域的先驱 Mission Bio Inc. 表示,AVROBIO 用于溶□体疾病的管道部署了一种单细胞转导测定。 使用 Mission Bio 的 Tapestri 平台,两家公司共同开发了一种准确且可重复的检测方法,用于测量数千个细胞样本中转导细胞的百分比。 在第 4 届基因治疗分析与开发 (GTAD) 峰会上,AVROBIO 展示了使用该测定法评估通过慢病毒载体自製的基因工程药物的转导率的结果。
同样,2022 年 6 月,英国创新机构 Innovate UK 向 Orbit Discovery Ltd. 授予了 Smart 赠款。 这家私人控股的生物製药公司专门使用专有的亲和力和基于细胞的功能筛选平台来识别□治疗候选药物。 这笔拨款总额为 472,000 英镑(约合 579,474 美元),将促进基于液滴的微流体技术在基于细胞的功能筛选中的应用,显着提高 Orbit □展示平台的容量和吞吐量。我们计划对其进行改进。 这种高通量筛选平台针对多膜蛋白,如 G 蛋白偶联受体,这些蛋白在相关生物学重要领域难以作为治疗靶点。
2022 年 7 月,为生物技术和生命科学行业提供最先进的多孔塑料材料和微孔板技术的製造商和开发商 PolveaScience 宣布推出 eGecko。 这种自动化条形码贴标系统提供了最佳的高通量解决方案,可将条形码标籤准确地贴在培养皿的板和架上。
此类发展有助于预测期内生物製药和生物技术公司的增长。
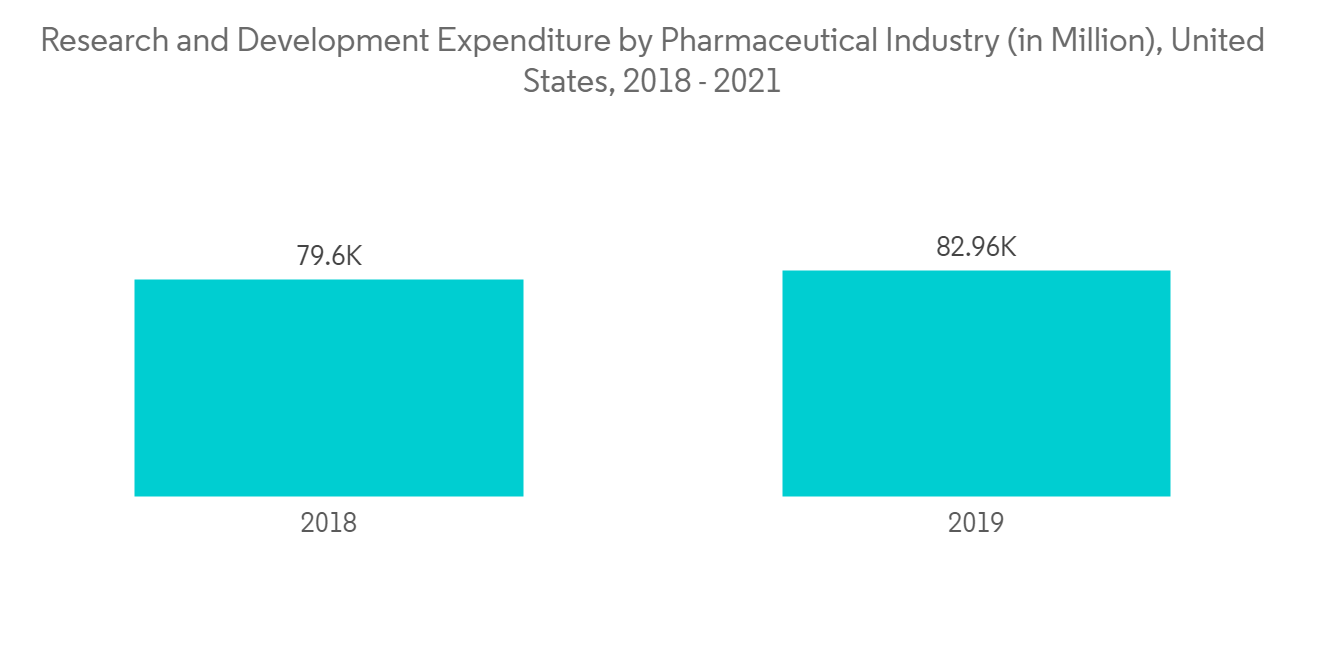
北美有望显着增长
北美的特点是可以轻鬆获得技术先进的产品、慢性病患病率增加、研发活动支出增加、该地区有领先公司以及完善的医疗保健基础设施。预计它将主导市场,因为等各种因素。
根据加拿大癌症协会 2022 年 5 月发表的一篇文章,到 2022 年底,估计将有 3,200 名加拿大人被诊断出患有脑癌和脊髓癌,其中包括 1,850 名男性和 1,350 名女性。 此外,如上述来源所述,每天有 27 名加拿大人被诊断出患有脑瘤。 估计有 55,000 名加拿大人患有脑瘤。 人群中脑肿瘤的高患病率强调了对创新疗法的需求。 这导致对药物发现和开发的需求增加,推动生物製药公司采用高通量工艺开发。
此外,本地公司的战略举措正在推动市场的增长。 例如,2022 年 9 月,Arsenal Biosciences, Inc. 与 Genentech 合作。 Genentech 将引入 ArsenalBio 的专有技术,用于 T 细胞的高通量工艺开发和工程,以确定基于 T 细胞的疗法中的关键成功迴路。 这一发现过程将通过融合自动化、大规模基因组工程和人工智能算法,帮助并加速下一代细胞疗法的设计、构建和测试。
此外,中国首家DNA高通量合成技术公司Dynegene Technologies亮相第二届美国人类遗传学大会(ASHG)。 公司表示,Dynegene Technologies的高通量DNA合成技术未来将进入美国市场,为海外用户提供高品质、高性价比的产品和服务。 它将大力支持合成生物学、生物医学工具和分子诊断材料等领域。 预计此类开发将加速为多个最终用户使用高通量工艺开发。 由于这些发展,预计北美地区在预测期内将出现显着增长。
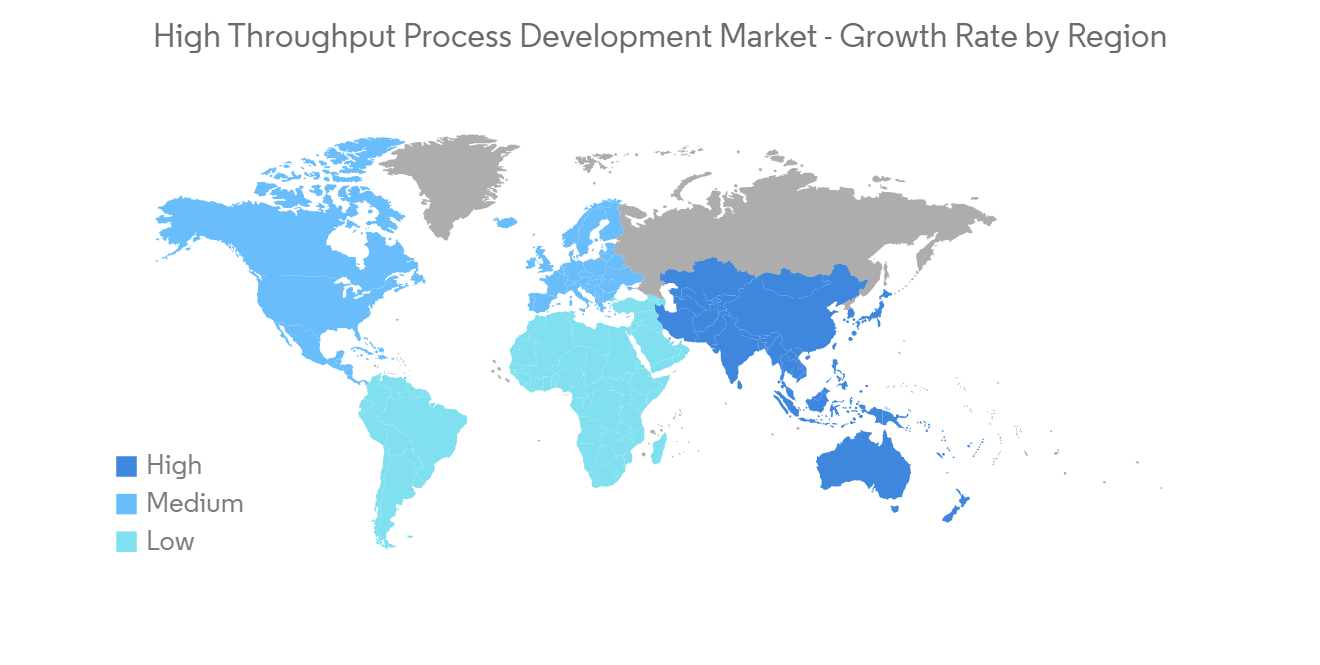
高通量工艺开发市场竞争对手分析
由于在全球和区域运营的公司的存在,高通量工艺开发市场正在经历一些整合和竞争。 竞赛其中包括Danaher Corporation、General Electric Company(GE Healthcare)、Thermo Fisher Scientific Inc.、Merck Millipore Limited、Agilent Technologies, Inc、Bio-Rad Laboratories, Inc、Eppendorf SE, Perkinelmer, Inc、Sartorius Stedim Biotech GmbH、Tecan Group AG,以及包括分析多家知名的国际和本地公司拥有市场份额,例如 Tecan Group AG。
其他福利。
- Excel 格式的市场预测 (ME) 表
- 3 个月的分析师支持
内容
第一章介绍
- 调查假设和市场定义
- 本次调查的范围
第二章研究方法论
第 3 章执行摘要
第四章市场动态
- 市场概览
- 市场驱动因素
- 启动针对新药物靶标的研发活动
- 降低生物製药和生物技术公司製造成本的压力越来越大
- 市场製约因素
- 先进技术的高成本和缺乏足够的基础设施
- 产业吸引力 - 波特五力分析
- 新进入者的威胁
- 买家的议价能力
- 供应商的议价能力
- 替代品的威胁
- 竞争对手之间的竞争强度
第 5 章市场细分(按价值划分的市场规模)
- 按产品/服务类型
- 消耗品
- 仪器
- 服务详情
- 软件
- 按技术
- 色谱法
- 紫外-可见光谱
- 其他技术
- 最终用户
- 生物製药和生物技术公司
- 委託研究机构
- 其他最终用户
- 按地区
- 北美
- 美国
- 加拿大
- 墨西哥
- 欧洲
- 德国
- 英国
- 法国
- 意大利
- 西班牙
- 其他欧洲
- 亚太地区
- 中国
- 日本
- 印度
- 澳大利亚
- 韩国
- 其他亚太地区
- 中东和非洲
- 海湾合作委员会
- 南非
- 其他中东和非洲地区
- 南美洲
- 巴西
- 阿根廷
- 其他南美洲
- 北美
第六章竞争格局
- 公司简介
- Danaher Corporation
- General Electric Company(GE Healthcare)
- Thermo Fisher Scientific Inc.
- Merck
- Agilent Technologies, Inc.
- Bio-Rad Laboratories, Inc.
- Eppendorf SE
- Perkinelmer, Inc.
- Sartorius Stedim Biotech GmbH
- Tecan Group AG
第七章市场机会与未来趋势
The high throughput process development market is expected to register a CAGR of 14.4% over the forecast period.
In its preliminary phase, the COVID-19 pandemic significantly impacted the high throughput process development (HTPD) market due to disruptions in research and development activities worldwide. As the pandemic continued worsening, it accelerated the vaccine and drug development process against the SARS-CoV-2 virus. As per the November 2022 update by BGI Genomics, an integrated solutions provider of precision medicine with the use of HTPD, upon the onset of the pandemic, the Institute of Molecular Genetic and Genetic Engineering (IMGGE) at the University of Belgrade immediately announced offering its expertise in molecular diagnostic methods. A partnership between BGI and IMGGE commenced with the construction of two permanent Huoyan laboratories. The first laboratory was established on the premises of the University Clinical Center of Serbia approximately 40 days after the pandemic was publicly disclosed. One of the two national laboratories for molecular diagnostics of infectious agents was established in Belgrade. By July 2020, the second National Laboratory, strategically positioned in the Serbian city of Nis to serve the region, was operational. Such developments contributed to the market's growth amid the pandemic.
In the post-pandemic phase, the market is expected to witness significant growth. For instance, as per the article published in September 2022 by Frontiers in Medical Technology, automated microfluidic technologies have considerable assurance for improving COVID-19 diagnostic test accessibility and speeding up high throughput detection techniques, specifically for POC testing. Additionally, COVID-19 diagnosis can easily be performed using the automated microfluidic platforms created for other pathogens. Thus, per the analysis, with the anticipated emergence of newer SARS-CoV-2 strains globally, the market is predicted to gain traction over the coming years.
Additionally, the growing need for identifying new drug targets, advancements in drug discovery and development technology, an increase in pharmaceutical research and development expenditure, and the benefits associated with HTPD in terms of cost reduction and process efficiency are major factors driving market growth in the coming years.
With high throughput screening, numerous samples can be examined simultaneously under the same circumstances. In addition, with robotic capabilities, hundreds and thousands of samples could be examined at once. The high throughput process development is speedier and more affordable for large sample sizes, making it a convenient option for screening newer drug targets for drug development. Such advantages offered by high throughput process development in the drug discovery process, where several potential drug candidates need to be screened, are expected to boost the market's growth shortly.
Moreover, a million human genomes were sequenced in 2020 due to high throughput sequencing technologies, revolutionizing genetics and genomics. As a result, the pharmaceutical industry now has access to a wide range of prospective therapeutic targets that demand additional investigation, driving the demand for high throughput process development.
Likewise, growing research and development in the pharmaceutical industry will contribute to the market's growth. For clinical trials of neurodegenerative diseases like Alzheimer's, drugs that target the central nervous system (CNS) have a historically high failure rate. Also, time consumption and costliness of in-vivo screening remain essential components of preclinical testing. High throughput technologies have recently made it possible for these tests better to meet the demands of the current pharmacological development pace. For instance, in June 2021, scientists from the University of Artois in France miniaturized a patented BBB in-vitro model, enabling automated high throughput compound screening. Robotic cell seeding, assay testing, and imaging were used to automate the model, which had previously been carried out manually using a 12-well format. Automation improved accuracy and decreased the time needed for assay execution by researchers. It significantly increased precision and reduced the time spent by researchers performing assays. Ultimately, this allowed more compounds to be screened in the early stages of drug discovery.
Furthermore, new product launches are contributing to the market's growth. For instance, in April 2021, Thermo Fisher Scientific received USFDA approval for high throughput and integrated COVID-19 Test Kit. Likewise, in November 2022, the FDA granted a EUA for Roche's COBAS MPXV high throughput test to detect mpox. Patients could immediately achieve the desired results with this high throughput solution in order to receive the right care as quickly as possible without being compelled to undergo needless additional testing or isolation. In December 2021, Becton, Dickinson, and Company's COR System was enhanced by adding a new MX instrument for high throughput molecular testing for infectious diseases.
Therefore, the studied market is expected to witness significant growth over the forecast period owing to the above-mentioned factors. However, the high cost of technology and the lack of infrastructure may impede the market's growth.
High Throughput Process Development Market Trends
Biopharmaceutical & Biotechnology Companies Segment Expected to Witness Significant Growth
The biopharmaceutical & biotechnology companies segment by the end-user is expected to witness significant growth over the analysis period. This is primarily attributed to the increase in R&D activities and spending by the companies and the emerging need to identify new drug targets for drug discovery.
Furthermore, significant factors augmenting the segment growth are a surge in the adoption of high throughput process development in these companies, the introduction of newer and advanced platforms, technological advancements in the fields of drug development, and growth in strategic developments by key players within the market. For instance, in November 2022, Mission Bio Inc., a pioneer in high-throughput single-cell DNA and multi-omics analysis, stated that AVROBIO's pipeline for lysosomal disorders deployed one single-cell transduction assay. Using Mission Bio's Tapestri Platform, the two firms worked together to create a precise, reproducible assay for measuring the proportion of transduced cells in samples made up of thousands of cells. At the 4th Annual Gene Therapy Analytical Development (GTAD) Summit, AVROBIO presented results on using this test to estimate the percentage transduction in autologous, lentiviral vector-mediated, genetically modified drug products.
Likewise, in June 2022, the United Kingdom's innovation agency, Innovate UK, gave a Smart grant to Orbit Discovery Ltd. This privately held biopharmaceutical firm specializes in identifying candidate peptide therapies using unique affinity and cell-based functional screening platforms. The funding, which has a total value of GBP 472,000 (~USD 579,474), will make it easier to use droplet-based microfluidics for cell-based functional screening and considerably increase the capacity and throughput of Orbit's peptide display platform. The high-throughput screening platform will target multi-membrane-spanning proteins such as G-protein-coupled receptors as difficult-to-access therapeutic targets in important biologically relevant areas.
In July 2022, Porvair Sciences, a manufacturer and developer of cutting-edge porous plastic materials and microplate technologies for the biotechnology and life science industries, introduced eGecko. This automated barcode application system offers the best high-throughput solution for accurately applying barcode labels onto racks of plates and petri dishes.
Such developments are contributing to the biopharmaceutical and biotechnology companies' growth over the forecast period.
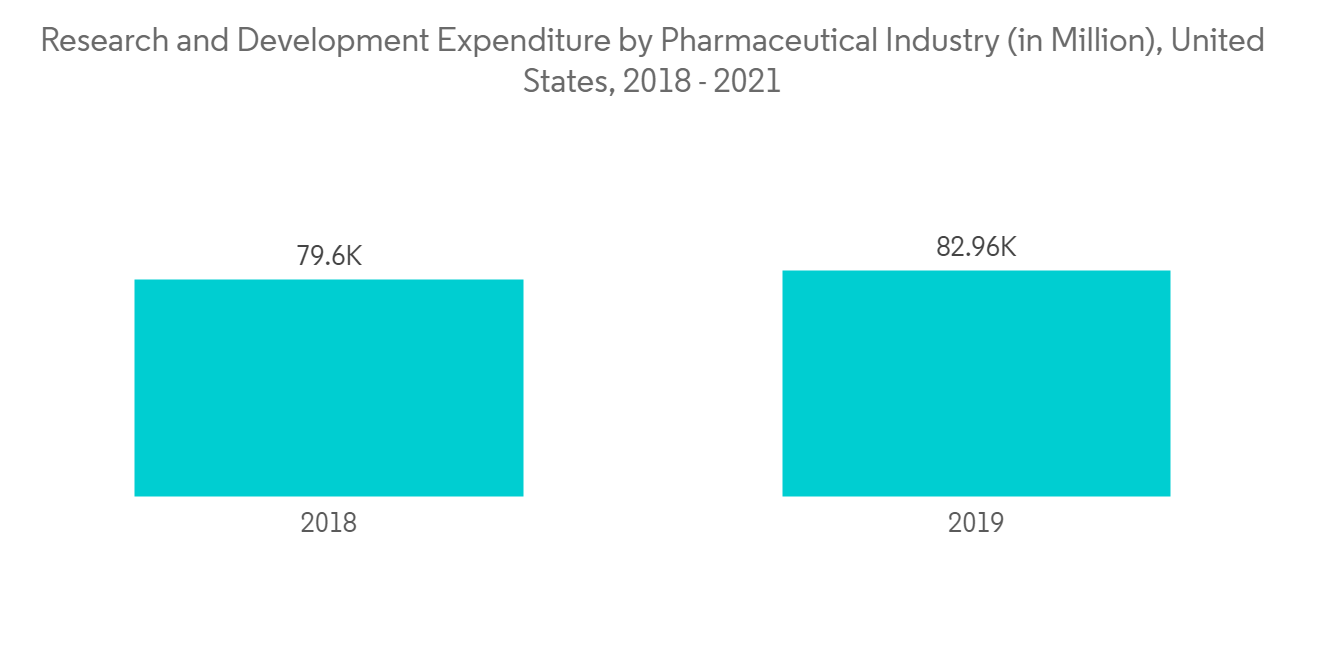
North America Expected to Witness Significant Growth
North America is expected to dominate the market owing to various factors, such as the easy availability of technologically advanced products, the growth in the prevalence of chronic diseases, higher spending on research and development activities, the presence of prominent players in the region, and well-established healthcare infrastructure.
According to the article published by the Canadian Cancer Society in May 2022, an estimated 3,200 Canadians will be diagnosed with brain and spinal cord cancer by the end of 2022, including 1,850 men and 1,350 women. In addition, as per the source above, 27 Canadians are diagnosed with brain tumors every day. It is estimated that 55,000 Canadians are surviving with brain tumors. The high prevalence of brain tumors among the target population underlines the need for innovative therapies. This drives demand for new drug discovery and development, in turn boosting the adoption of high throughput process development among biopharmaceutical companies.
Furthermore, strategic initiatives by the regional companies augment the market's growth. For instance, in September 2022, Arsenal Biosciences, Inc. collaborated with Genentech. Genentech will deploy ArsenalBio's proprietary technology for high throughput process development and engineering of T-cells to identify critical success circuits in T-cell-based therapies. This discovery process employs the convergence of automation, large-scale genome engineering, and artificial intelligence algorithms to aid and advance the design, building, and testing of next-generation cell therapies for cancer.
In addition, Dynegene Technologies, with the first DNA high throughput synthesis expertise in China, was featured at the 2nd American Annual Conference of Human Genetics (ASHG). The company stated that the high throughput DNA synthesis technology of Dynegene Technologies would enter the United States market in the future to deliver overseas users high-quality, cost-effective products and services. It will provide robust support for synthetic biology, biomedical tools, molecular diagnostic materials, and other fields. Such developments are meant to speed up the use of high throughput process development across several end-users. Thus, owing to such developments, the North American region is expected to witness significant growth over the forecast period.

High Throughput Process Development Market Competitor Analysis
The high throughput process development market is slightly consolidated and competitive due to the presence of companies operating globally and regionally. The competitive landscape includes an analysis of a few international as well as local companies that hold market shares and are well known, such as Danaher Corporation, General Electric Company (GE Healthcare), Thermo Fisher Scientific Inc., Merck Millipore Limited, Agilent Technologies, Inc., Bio-Rad Laboratories, Inc., Eppendorf SE, Perkinelmer, Inc., Sartorius Stedim Biotech GmbH, and Tecan Group AG.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions & Market Definition
- 1.2 Scope Of The Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET DYNAMICS
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Surge in Research and Development Activities for Newer Drug Targets
- 4.2.2 Growth in Pressure to Lower the Manufacturing Costs in Biopharmaceutical and Biotechnology Companies
- 4.3 Market Restraints
- 4.3.1 High Cost of Advanced Technologies and Lack of Adequate Infrastructure
- 4.4 Industry Attractiveness - Porter's Five Forces Analysis
- 4.4.1 Threat of New Entrants
- 4.4.2 Bargaining Power of Buyers
- 4.4.3 Bargaining Power of Suppliers
- 4.4.4 Threat of Substitute Products
- 4.4.5 Intensity of Competitive Rivalry
5 MARKET SEGMENTATION (Market Size by Value - USD million)
- 5.1 By Product and Services Type
- 5.1.1 Consumables
- 5.1.2 Instruments
- 5.1.3 Services
- 5.1.4 Software
- 5.2 By Technology
- 5.2.1 Chromatography
- 5.2.2 Ultraviolet-visible Spectroscopy
- 5.2.3 Other Technologies
- 5.3 By End-user
- 5.3.1 Biopharmaceutical and Biotechnology Companies
- 5.3.2 Contract Research Organizations
- 5.3.3 Other End-users
- 5.4 By Geography
- 5.4.1 North America
- 5.4.1.1 United States
- 5.4.1.2 Canada
- 5.4.1.3 Mexico
- 5.4.2 Europe
- 5.4.2.1 Germany
- 5.4.2.2 United Kingdom
- 5.4.2.3 France
- 5.4.2.4 Italy
- 5.4.2.5 Spain
- 5.4.2.6 Rest of Europe
- 5.4.3 Asia-Pacific
- 5.4.3.1 China
- 5.4.3.2 Japan
- 5.4.3.3 India
- 5.4.3.4 Australia
- 5.4.3.5 South Korea
- 5.4.3.6 Rest of Asia-Pacific
- 5.4.4 Middle East and Africa
- 5.4.4.1 GCC
- 5.4.4.2 South Africa
- 5.4.4.3 Rest of Middle East and Africa
- 5.4.5 South America
- 5.4.5.1 Brazil
- 5.4.5.2 Argentina
- 5.4.5.3 Rest of South America
- 5.4.1 North America
6 COMPETITIVE LANDSCAPE
- 6.1 Company Profiles
- 6.1.1 Danaher Corporation
- 6.1.2 General Electric Company (GE Healthcare)
- 6.1.3 Thermo Fisher Scientific Inc.
- 6.1.4 Merck
- 6.1.5 Agilent Technologies, Inc.
- 6.1.6 Bio-Rad Laboratories, Inc.
- 6.1.7 Eppendorf SE
- 6.1.8 Perkinelmer, Inc.
- 6.1.9 Sartorius Stedim Biotech GmbH
- 6.1.10 Tecan Group AG













