 |
市场调查报告书
商品编码
1627109
防伪包装:市场占有率分析、产业趋势、成长预测(2025-2030)Anti-Counterfeit Packaging - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030) |
||||||
※ 本网页内容可能与最新版本有所差异。详细情况请与我们联繫。
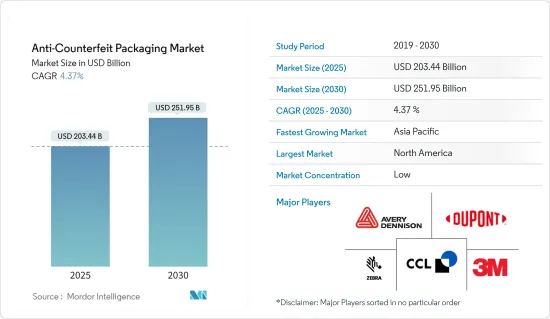
防伪包装市场规模预计到2025年为2034.4亿美元,预计到2030年将达到2519.5亿美元,预测期内(2025-2030年)复合年增长率为4.37%。
由于保护生命攸关产品的法规不断发展、防盗措施的技术进步、各种新出现的威胁以及对品牌保护的日益重视,防伪包装市场正在不断增长。
主要亮点
- 包括美国反假冒和盗版协议以及欧盟委员会的标籤和序列化倡议在内的监管措施预计将在预测期内加强新兴经济体的市场。
- 电子商务的蓬勃发展正在推动对防伪包装解决方案的需求。线上市场简化了造假者接触消费者的过程。为此,各品牌正在采用二维码和近距离场通讯(NFC) 标籤等高级身份验证功能,使客户能够在智慧型手机上验证产品的真伪。
- 政府和监管机构制定的严格法规进一步刺激了市场扩张。欧盟假药指令 (FMD) 要求所有处方药均配备唯一识别码和防篡改装置。中国有法规要求对某些产品类型实施追踪系统。这些要求正在刺激序列化技术和安全包装解决方案的创新。
- 根据 Macfarlane Group UK Ltd 2024 年 7 月报道,客製化防伪包装符合各行业的独特要求。製药业在保护其产品免受假冒威胁方面面临着巨大的挑战。为了解决这个问题,请考虑整合防伪包装功能,例如全像标籤、序列化二维码和识别条码。这些增强功能可防止篡改、加强产品认证并确保整个供应链中药品的完整性。
- 然而,由于安装防伪包装和技术创新的成本高昂,他们面临阻碍新进入者和潜在客户的挑战。如果没有足够的客户意识,这种犹豫以及条码和二维码等现有技术可以轻鬆复製正在阻碍市场扩张。
防伪包装市场趋势
药品和医疗保健推动市场需求
- 假药包装是全球非法仿製商品贸易中利润丰厚的领域之一。假药伤害甚至杀死了全世界数百万人。也对各大药品生产企业的品牌造成了严重损害。
- 已经发生多起向不同国家的人们注射假冒新冠肺炎 (COVID-19) 疫苗的事件。例如,世界卫生组织(WHO)发现了仿冒品的Covishield(由印度血清研究所製造),引起了世界各地相关人员的广泛关注。为了克服这种情况,世界各国政府都致力于制定法规来防止假药。
- 根据 Oliver 2024 年 9 月的一项研究,零售商越来越多地在药品标籤和包装上采取防伪措施。仿冒品可能会削弱消费者的信心并危及销售。例如,假药可能不含活性药物成分、可能无效或可能含有有害配方。
- 与此同时,製药业正在将防伪功能融入标籤和包装中。虽然这些技术不能完全防止犯罪分子篡改产品,但它们确实允许零售商验证药品包装并区分正品和恶意方製造的产品。
- 根据专门从事生命科学的资料分析、技术解决方案和临床研究服务领域的全球领导者 IQVIA 的数据,2020 年全球医药市场收入为 13,120 亿美元,预计 2023 年将达到 16,070 亿美元。 。因此,全球製药业收益的增加也可能增加预测期内对防伪包装的需求。
由于监管规定,北美保持压倒性的市场占有率
- 凭藉对研发坚定不移的承诺,北美预计在防伪包装市场中占据重要份额。美国联邦政府正在加强消除供应链中的仿冒品。透过利用 RFID 和 NFC 等先进包装技术,互联包装正成为保护消费者仿冒品侵害的重要解决方案。
- 随着消费者对透明度的需求增加,产品的可追溯性也随之增加。特别是在美国,执法机构每年都要应对经济合作暨发展组织(OECD) 查获的价值超过 1 兆美元的假货。日益严格的防伪法规正在进一步提高市场占有率。
- 为了满足这种不断增长的需求,製造商正在将创新技术融入其设备中,以更好地识别和追踪产品。此外,Astra Zeneca预计,到 2025 年,北美地区的销售额将达到 7,060 亿美元,使该地区成为造假者的有吸引力的目标。然而,防伪包装市场已趋成熟并有望成长。
- 此外,假冒行为透过滥用与商标相关的商誉和品质来损害品牌声誉。对于容易受到此类行为影响的品牌来说,法律保护对于保护其在加拿大市场中来之不易的声誉至关重要。援助请求 (RFA) 计划允许註册商标所有者对假货进口商提出法律质疑,这一过程将在加拿大边境服务局 (CBSA) 发现后启动。
- 不断增加的品牌保护力度和政府的大力支持正在推动北美防伪包装市场的发展。然而,有限的研发投资和高昂的设置成本的挑战可能会限制该地区的市场扩张。
防伪包装产业概况
防伪包装市场较为分散。就市场占有率,例如艾利丹尼森公司、CCL工业公司、3M公司、EI杜邦公司和斑马技术公司。然而,凭藉创新和永续的包装,许多公司正在透过赢得新合约和开闢新市场来扩大其市场份额。
其他好处:
- Excel 格式的市场预测 (ME) 表
- 3 个月分析师支持
目录
第一章简介
- 研究假设和市场定义
- 调查范围
第二章调查方法
第三章执行摘要
第四章市场洞察
- 市场概况
- 产业吸引力-波特五力分析
- 供应商的议价能力
- 买方议价能力
- 新进入者的威胁
- 替代品的威胁
- 竞争公司之间敌对关係的强度
- 产业价值链分析
第五章市场动态
- 市场驱动因素
- 蓬勃发展的电子商务产业
- 製造商对品牌保护的兴趣日益浓厚
- 市场限制因素
- 防伪包装初始成本较高
- 技术简介
第六章 市场细分
- 依技术
- 追踪与追踪
- 防篡改
- 隐藏
- 隐藏
- 法医标记
- 按最终用户产业
- 饮食
- 医疗保健/製药
- 工业/汽车
- 家用电子产品
- 其他最终用户产业
- 按地区
- 北美洲
- 美国
- 加拿大
- 欧洲
- 英国
- 德国
- 法国
- 义大利
- 西班牙
- 亚洲
- 中国
- 印度
- 日本
- 澳洲/纽西兰
- 拉丁美洲
- 巴西
- 阿根廷
- 中东和非洲
- 南非
- 阿拉伯聯合大公国
- 北美洲
第七章 竞争格局
- 公司简介
- Avery Dennison Corporation
- CCL Industries Inc.
- 3M Company
- EI Du Pont De Nemours and Company
- Zebra Technologies Corporation
- Sicpa Holding SA
- AlpVision SA
- Applied Dna Sciences Inc.
- Uflex Limited
- Authentix Inc.
- Ampacet Corporation
- PharmaSecure Inc.
第八章投资分析
第九章 市场机会及未来趋势
The Anti-Counterfeit Packaging Market size is estimated at USD 203.44 billion in 2025, and is expected to reach USD 251.95 billion by 2030, at a CAGR of 4.37% during the forecast period (2025-2030).

The anti-counterfeit packaging market is witnessing growth, driven by evolving regulations to safeguard life-critical products, technological strides in anti-theft measures, a diverse array of emerging threats, and an increasing emphasis on brand protection.
Key Highlights
- Regulatory measures, including the U.S.'s Anti-counterfeiting Trade Agreement and the EU Commission's labeling and serialization initiatives, are poised to bolster the market in developed economies during the forecast period.
- The surge of e-commerce has heightened the demand for anti-counterfeit packaging solutions. Online marketplaces have simplified counterfeiters' access to consumers. In response, brands are adopting advanced authentication features, like QR codes and near-field communication (NFC) tags, enabling customers to verify product authenticity via smartphones.
- Stringent regulations set by governments and regulatory bodies further fuel the market's expansion. Take the EU's Falsified Medicines Directive (FMD), which mandates unique identifiers and anti-tampering devices for all prescription medications. In China, regulations enforce track-and-trace systems for select product categories. Such mandates have catalyzed innovations in serialization technologies and secure packaging solutions.
- According to Macfarlane Group UK Ltd, in July 2024, customized anti-counterfeit packaging caters to the unique requirements of various industries. The pharmaceutical sector grapples with formidable challenges in safeguarding its products from counterfeit threats. To counteract this, consider integrating anti-counterfeit packaging features such as holographic labels, serialized QR codes, and distinct barcodes. These enhancements deter tampering and bolster product authentication, ensuring the pharmaceuticals' integrity throughout the supply chain.
- However, the market faces challenges owing to the high costs associated with setting up and innovating anti-counterfeit packaging, which deters new entrants and potential clients. Without adequate customer awareness, this hesitance and the ease of replicating existing technologies like barcodes and QR codes hamper market expansion.
Anti-Counterfeit Packaging Market Trends
Pharmaceuticals and Healthcare to Drive the Market Demand
- Counterfeit packaging for pharmaceuticals is one of the lucrative sectors of the global trade in illegally copied goods. Fake drugs harm and even kill millions of people across the world. It inflicts severe damage on the brand names of big pharmaceutical manufacturers.
- Several incidents of fake COVID-19 vaccines were administered to the populations of various countries. For example, the World Health Organization (WHO) discovered counterfeit versions of Covishield (manufactured by the Serum Institute of India), which caused widespread concern among stakeholders worldwide. To overcome this, governments across regions focused on implementing regulations to prevent counterfeit medicines.
- According to a study by Oliver Inc. in September 2024, retailers are increasingly adopting anti-counterfeiting measures in pharmaceutical labeling and packaging. Counterfeit products can erode consumer trust and risk sales. Counterfeit medicines, for instance, might lack active pharmaceutical ingredients, possess ineffective qualities, or even contain harmful formulations.
- On a positive note, the pharmaceutical sector has integrated anti-counterfeiting features into its labeling and packaging. While these technologies can not entirely prevent criminals from altering products, they empower retailers to authenticate pharmaceutical packaging, distinguishing between genuine items and those crafted by malicious entities.
- According to IQVIA, a global leader, delivers data analytics, technology solutions, and clinical research services tailored for the life sciences sector, revenue of global pharmaceutical market in 2020 was USD 1,312 billion and it has reached USD 1,607 billion in 2023. Therefore, rise in revenue of pharmaceutical sector globally will also rise the demand for anti-countefeit packaging over the forecast period.
Regulations Have Enabled North America to Maintain a Dominant Market Share
- North America is poised to command a significant share of the anti-counterfeit packaging market, bolstered by its unwavering commitment to research and development. The U.S. Federal Government is intensifying efforts to purge counterfeit goods from its supply chain. Leveraging advanced technologies like RFID and NFC, connected packaging emerges as a pivotal solution in safeguarding consumers from counterfeit products.
- As consumer demand for transparency surges, so does the traceability of products. Notably, the U.S. grapples with counterfeit goods valued over USD 1 trillion annually, derived from law enforcement seizures and reported by the Organization for Economic Cooperation and Development. Strengthened anti-counterfeit regulations have further bolstered the market's share.
- In response to this escalating demand, manufacturers embed innovative technologies into their equipment, enhancing product identification and tracking. Furthermore, with projections from AstraZeneca estimating North American sales at USD 706 billion by 2025, the region becomes a prime target for counterfeiters. However, the anti-counterfeit packaging market is set for growth, given its maturity.
- Further, counterfeiting undermines a brand's reputation by exploiting the goodwill and quality associated with its trademark. Legal protections are vital for brands susceptible to such practices to safeguard their hard-earned reputation in the Canadian market. The Request for Assistance (RFA) program empowers registered trademark owners to legally challenge importers of counterfeit goods, a process initiated upon detection by the Canadian Border Services Agency (CBSA).
- Heightened brand protection efforts and robust government backing fuel the North American anti-counterfeit packaging market. However, challenges loom: limited R&D investments and the burden of high setup costs could temper the market's expansion in the region.
Anti-Counterfeit Packaging Industry Overview
The anti-counterfeit packaging market is fragmented. A few major players, such as Avery Dennison Corporation, CCL Industries Inc., 3M Company, E.I. Du Pont De Nemours and Company, Zebra Technologies Corporation, and others, dominate the market in terms of market share. However, with innovative and sustainable packaging, many companies are increasing their market presence by securing new contracts and tapping new markets.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET INSIGHTS
- 4.1 Market Overview
- 4.2 Industry Attractiveness - Porter's Five Forces Analysis
- 4.2.1 Bargaining Power of Suppliers
- 4.2.2 Bargaining Power of Buyers
- 4.2.3 Threat of New Entrants
- 4.2.4 Threat of Substitute Products
- 4.2.5 Intensity of Competitive Rivalry
- 4.3 Industry Value Chain Analysis
5 MARKET DYNAMICS
- 5.1 Market Drivers
- 5.1.1 Booming E-commerce Industry
- 5.1.2 Increasing Focus of Manufacturers on Brand Protection
- 5.2 Market Restraint
- 5.2.1 High-initial Costs of Anti-Counterfeit Packaging
- 5.3 Technology Snapshot
6 MARKET SEGMENTATION
- 6.1 By Technology
- 6.1.1 Trace and Track
- 6.1.2 Tamper-evident
- 6.1.3 Covert
- 6.1.4 Overt
- 6.1.5 Forensic Markers
- 6.2 By End-User Industries
- 6.2.1 Food and Beverage
- 6.2.2 Healthcare and Pharmaceuticals
- 6.2.3 Industrial and Automotive
- 6.2.4 Consumer Electronics
- 6.2.5 Other End User Industries
- 6.3 By Geography
- 6.3.1 North America
- 6.3.1.1 United States
- 6.3.1.2 Canada
- 6.3.2 Europe
- 6.3.2.1 United Kingdom
- 6.3.2.2 Germany
- 6.3.2.3 France
- 6.3.2.4 Italy
- 6.3.2.5 Spain
- 6.3.3 Asia
- 6.3.3.1 China
- 6.3.3.2 India
- 6.3.3.3 Japan
- 6.3.4 Australia and New Zealand
- 6.3.5 Latin America
- 6.3.5.1 Brazil
- 6.3.5.2 Argentina
- 6.3.6 Middle East & Africa
- 6.3.6.1 South Africa
- 6.3.6.2 United Arab Emirates
- 6.3.1 North America
7 COMPETITIVE LANDSCAPE
- 7.1 Company Profiles
- 7.1.1 Avery Dennison Corporation
- 7.1.2 CCL Industries Inc.
- 7.1.3 3M Company
- 7.1.4 E.I. Du Pont De Nemours and Company
- 7.1.5 Zebra Technologies Corporation
- 7.1.6 Sicpa Holding SA
- 7.1.7 AlpVision SA
- 7.1.8 Applied Dna Sciences Inc.
- 7.1.9 Uflex Limited
- 7.1.10 Authentix Inc.
- 7.1.11 Ampacet Corporation
- 7.1.12 PharmaSecure Inc.











