 |
市场调查报告书
商品编码
1644450
疫苗物流:市场占有率分析、产业趋势与统计、成长预测(2025-2030 年)Vaccine Logistics - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030) |
||||||
※ 本网页内容可能与最新版本有所差异。详细情况请与我们联繫。
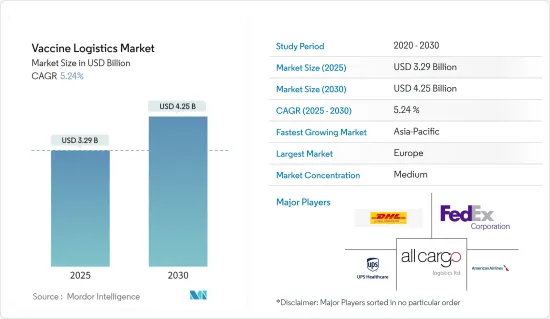
疫苗物流市场规模在 2025 年估计为 32.9 亿美元,预计到 2030 年将达到 42.5 亿美元,预测期内(2025-2030 年)的复合年增长率为 5.24%。
新型疫苗、不断发展的疫苗接种时间表、创新的服务提供策略、不断扩大的目标人口、不断增长的低温运输基础设施需求以及有限的资金正在重塑疫苗运输市场的动态。
气候变迁正在大幅改变感染疾病的性质。气温升高扩大了蚊子、蜱虫等病媒的栖息地,助长了疟疾、登革热和莱姆病等疾病的传播。这些模式,加上极端天气感染疾病和生态干扰,增加了通用、水传播和呼吸道感染疾病的风险,感染疾病加剧了对疫苗的需求。
例如,辉瑞-BioNTech 疫苗需要-112°F 至-76°F(-80°C 至-60°C)的超低温,并且必须存放在专用的温控热感运输箱中。同样,Moderna 疫苗对温度的要求也不像辉瑞疫苗那么极端,后者必须储存在 -4°F (-20°C) 的环境中。疫苗必须从生产基地直接运送到使用点,避免长时间暴露在高温下。
运送对温度敏感的疫苗是最具挑战性的医药产品之一。这些关键产品需要在整个供应链中小心处理,并依赖精确协调、温控的物流。保持恆定的温度至关重要,疫苗从生产到管理都必须保持在指定的范围内。任何偏离该范围的行为都会损害疫苗的功效及其预防目标疾病的能力。
此外,由于疫苗要求的不断变化、气候变迁的影响以及严格的温度控制的需要,疫苗运输市场面临许多挑战。应对这些挑战对于确保全球疫苗的有效性和安全性至关重要。
疫苗物流市场趋势
北美疫苗物流市场的成长与转型
北美疫苗物流市场正在经历成长,主要受温控运输和储存解决方案需求激增的推动。物流供应商正在加强其低温运输能力,以确保疫苗在运输过程中保持其有效性。例如,联邦快递扩大了其在美国范围内的温控设施网络,以便高效处理符合严格温度规定的疫苗。这项策略性倡议不仅解决了物流挑战,而且透过费城和达拉斯等城市的关键设施显着增强了我们的冷链能力。
此外,新疫苗的推出和疫苗接种时间表的改变正在显着改变北美的物流格局。 2024年,XPO部署了一辆热敏疫苗运输车,监督全国热敏疫苗的分发。该公司已成功向芝加哥和休士顿等主要城市完成疫苗配送,确保疫苗从源头到医护人员的运输过程均保持在规定的温度范围内。
总之,在低温运输技术进步和新疫苗推出的推动下,北美疫苗物流市场正在快速发展。这些公司也确保全部区域疫苗分发高效、可靠。
疫苗物流服务的低温运输技术创新
首先,对低温运输解决方案的需求日益增加。从2024年开始的十年间,低温运输物流领域的投资激增。根据《生物製药低温运输原始资料》报道,2020 年,温控物流约占生物物流支出的 18%。没有迹象显示这种成长趋势将会停止。
例如,许多疫苗不耐热,如白喉、破伤风和百日咳 (DTP) 疫苗以及麻疹、腮腺炎和德国麻疹(MMR) 疫苗。这些对热敏感的疫苗必须保存在2°C至8°C的温度下,否则它们会因其生物成分而迅速降解。因此,他们严重依赖多级冷藏或低温运输配送系统。
此外,随着人工智慧和区块链等先进技术的出现,製药业正经历着供应链可视性提高的一个重要趋势。现在,追踪、监控和管理温度敏感产品会产生比以往更多的资料。这种增强可视性的技术不仅可以降低腐败风险,还能确保遵守监管标准。
此外,公司正在创新具有密封温控系统的高科技容器。这些货柜促进了货仓和飞机之间温度敏感货物的无缝运输,特别满足了製药业的需求。
例如,英国泰恩港正在部署支援5G的自主无人机。这些无人机将提高业务效率,监督货物处理并加快流程,加强低温运输并减少对温度敏感的物资的延误。
总之,对低温运输解决方案日益增长的需求加上技术进步正在显着改变疫苗物流格局。提高供应链可视性和创新的温度控制系统对于确保疫苗安全有效分发至关重要。
疫苗物流行业概况
疫苗物流市场较为分散,主要由 DHL Global Forwarding、AllCargo Logistics、美国航空、联邦快递、UPS Healthcare 等国际公司主导。这些大公司主要透过收购来推行扩大策略。与小公司相比,他们的现有业务使得市场扩张更加顺利。
冷藏、加急配送服务和散装疫苗运输的需求正在上升。政府投资的活性化进一步推动了这一成长,为市场参与者提供了扩大影响力和提高效率的机会。
其他福利:
- Excel 格式的市场预测 (ME) 表
- 3 个月的分析师支持
目录
第 1 章 简介
- 调查结果
- 调查前提
- 研究范围
第二章调查方法
- 分析方法
- 研究阶段
第三章执行摘要
第四章 市场动态与洞察
- 当前市场状况
- 市场动态
- 驱动程式
- 温控包装的创新
- 跨国合作及加强医疗基础设施的倡议
- 限制因素
- 供应链中断和运输瓶颈可能会阻碍疫苗的及时分发。
- 监理与合规挑战
- 机会
- 区块链和物联网技术的采用将提高透明度和可追溯性。
- 下一代疫苗
- 驱动程式
- 产业吸引力-波特五力分析
- 供应商的议价能力
- 消费者/购买者的议价能力
- 新进入者的威胁
- 替代品的威胁
- 竞争对手之间的竞争
- 技术趋势和自动化
- 政府法规和倡议
- 产业价值链/供应链分析
- 专注于环境和温度控制存储
- 地缘政治与疫情将如何影响市场
第五章 市场区隔
- 按服务
- 运输
- 陆路(公路和铁路)
- 航空
- 海上运输
- 仓库
- 附加价值服务(包装、标籤等)
- 运输
- 按最终用户
- 医院
- 药品製造商和经销商
- 其他最终使用者(血库、诊所等)
- 按地区
- 亚太地区
- 中国
- 日本
- 澳洲
- 印度
- 新加坡
- 马来西亚
- 印尼
- 泰国
- 韩国
- 其他亚太地区
- 欧洲
- 德国
- 法国
- 英国
- 义大利
- 其他欧洲国家
- 北美洲
- 美国
- 加拿大
- 墨西哥
- 南美洲
- 巴西
- 哥伦比亚
- 阿根廷
- 南美洲其他地区
- 中东
- 埃及
- 卡达
- 沙乌地阿拉伯
- 阿拉伯聯合大公国
- 其他中东地区
- 亚太地区
第六章 竞争格局
- 市场集中度概览
- 公司简介
- DHL Global Forwarding
- AllCargo Logistics
- American Airlines
- DB Schenker
- FedEx Corporation
- Kuehne Nagel
- Nippon Express
- Yamato Logistics
- Americold Logistics
- lynden international logistics
- DP World
- Coldman Logistics
- Cavalier Logistics*
- 其他公司
第七章:市场的未来
第 8 章 附录
- 宏观经济指标(GDP分布,依活动划分)
- 经济统计 - 运输及仓储业对经济的贡献
- 对外贸易统计 - 按商品、目的地和原产国分類的进出口数据
The Vaccine Logistics Market size is estimated at USD 3.29 billion in 2025, and is expected to reach USD 4.25 billion by 2030, at a CAGR of 5.24% during the forecast period (2025-2030).

New vaccines, evolving immunization schedules, innovative service delivery strategies, a broader target population, heightened cold-chain infrastructure demands, and limited funding are reshaping the dynamics of the vaccine transportation market.
Climate change has significantly altered the landscape of infectious diseases. Rising temperatures are broadening the habitats of disease vectors like mosquitoes and ticks, facilitating the spread of diseases such as malaria, dengue fever, and Lyme disease. This pattern, alongside extreme weather events and ecosystem disruptions, heightens the risk of zoonotic diseases, waterborne illnesses, and respiratory infections, underscoring the growing demand for vaccines.
For example, the Pfizer-BioNTech Vaccine mandates storage in specialized temperature-controlled thermal shippers, requiring ultra-low temperatures between -112°F and -76°F (-80°C to -60°C). Similarly, while the Moderna Vaccine doesn't demand the extreme temperatures of its Pfizer counterpart, it still requires storage at -4°F (-20°C). This vaccine must be transported directly from the manufacturing facility to its point of use, avoiding any prolonged exposure to elevated temperatures.
Transporting temperature-sensitive vaccines stands out as a particularly challenging endeavor among pharmaceuticals. These vital products necessitate meticulous handling throughout the supply chain, relying on precisely coordinated temperature-controlled logistics. Maintaining a consistent temperature is crucial, vaccines must remain within a specified range from production to administration. Deviating from this range jeopardizes the vaccine's potency and its protective efficacy against targeted diseases.
Moreover, the vaccine transportation market faces numerous challenges due to evolving vaccine requirements, climate change impacts, and stringent temperature control needs. Addressing these challenges is crucial to ensuring the efficacy and safety of vaccines worldwide.
Vaccine Logistics Market Trends
Growth and Transformation in the North American Vaccine Logistics Market
The North American vaccine logistics market is witnessing growth, primarily fueled by the surging demand for temperature-controlled transportation and storage solutions. Logistic providers are bolstering their cold chain capabilities to ensure vaccines retain their efficacy during transit. For example, FedEx has broadened its network of temperature-controlled facilities throughout the U.S., facilitating the efficient handling of vaccines that adhere to stringent temperature regulations. This strategic move not only addresses logistical challenges but also significantly enhances its cold chain capabilities, with key facilities in cities like Philadelphia and Dallas.
Furthermore, the rollout of new vaccines and the shifting immunization schedule are transforming North America's logistics landscape. In 2024, XPO has rolled out its thermally mapped transportation fleet to oversee the distribution of heat-sensitive vaccines nationwide. The company has successfully orchestrated deliveries in major cities like Chicago and Houston, guaranteeing that vaccines stay within the mandated temperature ranges from their origin to healthcare providers.
In conclusion, the North American vaccine logistics market is evolving rapidly, driven by advancements in cold chain technology and the introduction of new vaccines. Companies are also ensuring efficient and reliable vaccine distribution across the region.
Cold Chain Innovations in Vaccine Logistics Services
First, the demand for cold chain solutions is on the rise. Over the past decade from 2024, investments in the cold chain logistics sector have surged. As reported by the Biopharma Cold Chain Sourcebook, in 2020, temperature-controlled logistics made up nearly 18% of biopharma logistics expenditures. This upward trend shows no signs of slowing down.
For instance, many vaccines, such as those for diphtheria, tetanus, pertussis (DTP), and measles, mumps, and rubella (MMR), lack thermal stability. These heat-sensitive vaccines, if not kept between 2°C and 8°C, degrade quickly due to their biological components. Consequently, they depend heavily on a multi-stage refrigerated or cold-chain distribution system.
Moreover, with the advent of advanced technologies like artificial intelligence (AI) and blockchain, the pharmaceutical sector is witnessing a crucial trend: enhanced supply chain visibility. The tracking, monitoring, and management of temperature-sensitive products now generate more data than ever. Technologies that bolster this visibility not only mitigate spoilage risks but also ensure adherence to regulatory standards.
In addition, companies are innovating high-tech containers equipped with closed temperature-controlled systems. These containers facilitate the seamless transport of temperature-sensitive goods between cargo warehouses and aircraft, specifically catering to the pharmaceutical sector.
For instance, at the Port of Tyne in the UK, 5G-enabled autonomous drones have been deployed. These drones boost operational efficiency and oversee cargo handling, bolstering the cold chain by expediting processes and reducing delays for temperature-sensitive supplies.
In conclusion, the increasing demand for cold chain solutions, coupled with technological advancements, is transforming the vaccine logistics landscape. Enhanced supply chain visibility and innovative temperature-controlled systems are critical in ensuring the safe and efficient distribution of vaccines.
Vaccine Logistics Industry Overview
The vaccine logistics market is fragmented and is dominated by international companies, such as DHL Global Forwarding, AllCargo Logistics, American Airlines, FedEx Corporation and UPS Healthcare . These giants are pursuing expansion strategies, primarily through acquisitions. Their established presence allows for smoother market expansion compared to smaller players.
The demand for refrigerated warehouses, expedited delivery services, and bulk vaccine transportation is on the rise. This surge is further bolstered by heightened government investments, offering market players a chance to broaden their reach and enhance efficiency over time.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Deliverables
- 1.2 Study Assumptions
- 1.3 Scope of the Study
2 RESEARCH METHODOLOGY
- 2.1 Analysis Methodology
- 2.2 Research Phases
3 EXECUTIVE SUMMARY
4 MARKET INSIGHTS AND DYNAMICS
- 4.1 Current Market Scenario
- 4.2 Market Dynamics
- 4.2.1 Drivers
- 4.2.1.1 Technology innovation in temperature controlled packaging
- 4.2.1.2 Cross Border collaborations and initiative to enhance healthcare infrastructure
- 4.2.2 Restraints
- 4.2.2.1 Supply chain distruption and transportation bottlenecks can hinder timely vaccine distribution
- 4.2.2.2 Regulatory and Compiliance Challenges
- 4.2.3 Opportunities
- 4.2.3.1 Adoption of blockchain and IoT technology can improve transparency and tracebility
- 4.2.3.2 Next-Generation Vaccines
- 4.2.1 Drivers
- 4.3 Industry Attractiveness - Porter's Five Forces Analysis
- 4.3.1 Bargaining Power of Suppliers
- 4.3.2 Bargaining Power of Consumers/Buyers
- 4.3.3 Threat of New Entrants
- 4.3.4 Threat of Substitute Products
- 4.3.5 Intensity of Competitive Rivalry
- 4.4 Technological Trends and Automation
- 4.5 Government Regulations and Initiatives
- 4.6 Industry Value Chain/Supply Chain Analysis
- 4.7 Spotlight on Ambient/Temperature-controlled Storage
- 4.8 Impact of Geopolitics and Pandemic on the Market
5 MARKET SEGMENTATION
- 5.1 By Service
- 5.1.1 Transportation
- 5.1.1.1 Land (Road and Rail)
- 5.1.1.2 Air
- 5.1.1.3 Sea
- 5.1.2 Warehousing
- 5.1.3 Value-added Services (Packaging, Labeling, etc.)
- 5.1.1 Transportation
- 5.2 By End User
- 5.2.1 Hospitals
- 5.2.2 Drug Manufacturers and Distributors
- 5.2.3 Other End Users (Blood Banks, Clinics, etc.)
- 5.3 By Geography
- 5.3.1 Asia-Pacific
- 5.3.1.1 China
- 5.3.1.2 Japan
- 5.3.1.3 Australia
- 5.3.1.4 India
- 5.3.1.5 Singapore
- 5.3.1.6 Malaysia
- 5.3.1.7 Indonesia
- 5.3.1.8 Thailand
- 5.3.1.9 South Korea
- 5.3.1.10 Rest of Asia-Pacific
- 5.3.2 Europe
- 5.3.2.1 Germany
- 5.3.2.2 France
- 5.3.2.3 United Kingdom
- 5.3.2.4 Italy
- 5.3.2.5 Rest of Europe
- 5.3.3 North America
- 5.3.3.1 United States
- 5.3.3.2 Canada
- 5.3.3.3 Mexico
- 5.3.4 South America
- 5.3.4.1 Brazil
- 5.3.4.2 Colombia
- 5.3.4.3 Argentina
- 5.3.4.4 Rest of South America
- 5.3.5 Middle East
- 5.3.5.1 Egypt
- 5.3.5.2 Qatar
- 5.3.5.3 Saudi Arabia
- 5.3.5.4 United Arab Emirates
- 5.3.5.5 Rest of the Middle East
- 5.3.1 Asia-Pacific
6 COMPETITIVE LANDSCAPE
- 6.1 Market Concentration Overview
- 6.2 Company Profiles
- 6.2.1 DHL Global Forwarding
- 6.2.2 AllCargo Logistics
- 6.2.3 American Airlines
- 6.2.4 DB Schenker
- 6.2.5 FedEx Corporation
- 6.2.6 Kuehne Nagel
- 6.2.7 Nippon Express
- 6.2.8 Yamato Logistics
- 6.2.9 Americold Logistics
- 6.2.10 lynden international logistics
- 6.2.11 DP World
- 6.2.12 Coldman Logistics
- 6.2.13 Cavalier Logistics*
- 6.3 Other Companies
7 FUTURE OF THE MARKET
8 APPENDIX
- 8.1 Macroeconomic Indicators (GDP Distribution, by Activity)
- 8.2 Economic Statistics - Transport and Storage Sector Contribution to Economy
- 8.3 External Trade Statistics - Exports and Imports by Product and by Country of Destination/Origin









![医疗保健分销市场- 按类型(药品[OTC、仿製药、品牌药]、生物製药[疫苗、单克隆抗体] 和医疗器械分销服务)、最终用途(医院、零售药房) - 全球预测(2024 - 2032 )](/sample/img/cover/42/default_cover_gmi.png)