 |
市场调查报告书
商品编码
1844477
残留物检测:市场占有率分析、产业趋势、统计数据和成长预测(2025-2030 年)Residue Testing - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030) |
||||||
※ 本网页内容可能与最新版本有所差异。详细情况请与我们联繫。
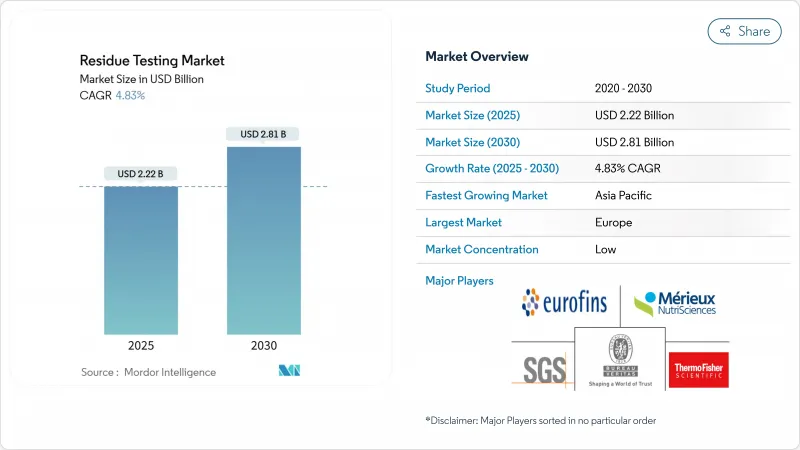
预计到 2025 年,食品残留检测市场收益将达到 22.2 亿美元,到 2030 年将扩大到 28.1 亿美元,复合年增长率为 4.83%。
成长的动力来自全球最大残留基准值收紧、技术的快速升级以及消费者对食品纯度不断增长的需求。根据欧洲食品安全局 2023 年的年度报告,99% 的食品样本符合欧盟法规,但 2% 的食品样本超过最大残留基准值,凸显了对强大检测基础设施的迫切需求。政府收紧法规的速度超过了大多数生产商的适应速度,将强制性检测范围扩大到新的残留类别,并要求对整个进口和国内供应链进行即时监控。质谱、定序和生物感测器平台的融合正在缩短分析週期,而食品召回的加速成长使检测需求保持在结构性的高位。食品残留检测市场仍然分散,随着服务提供者寻求在设备投资、数据分析和地理覆盖方面获得规模优势,这为有意义的整合创造了空间。
全球残留检测市场趋势与洞察
严格的全球食品安全法规
随着各国政府实施更严格的残留限量并扩大监测范围,日益严格的法规加速了检测需求。欧盟法规 2023/915 的实施设立了全面的污染物阈值,FDA 更新后的霉菌毒素监测计画现在包括使用多霉菌毒素分析方法的 T-2/HT-2 毒素和Zearalenone。中国在 2024 年发布了 47 项新的国家食品安全标准,其中包括七种专用检测方法,显示监管与国际最佳实践趋同。台湾于 2024 年 7 月引入了宠物食品中五种霉菌毒素(包括呕吐毒素和Fumonisins)的新安全耐受水平,标誌着监管范围扩大到人类消费之外。 FDA 食品分析实验室认可 (LAAF) 计划下的强制性霉菌毒素检测要求将于 2024 年 12 月开始实施,这将对认可的检测能力产生结构性需求。巴西采用新的食品法规结构并修订其食品接触金属技术法规,进一步体现了全球监管的协调一致,从而推动了系统性检测要求的提升。这些新兴市场的监管发展要求增加对检测基础设施和分析能力的投资。国际标准的协调一致为检测服务提供者创造了机会,同时确保了全球供应链中食品安全实践的一致性。
洁净标示食品和饮料的需求激增
消费者对透明、低加工食品的偏好促使製造商实施全面的残留检测通讯协定,以检验清洁标籤的声明。这一趋势对高端食品行业影响尤为显着,因为该行业的品牌以经过认证的纯度水平和不含合成残留物为特色。清洁标籤运动的范围已超越农药,涵盖重金属、加工助剂和包装污染物,从而扩大了产品特定检测的范围。零售商越来越要求第三方检验清洁标籤声明,这催生了对整个供应链进行系统性检测的需求。洁净标示标籤产品的高昂价格证明了增加检测投入的合理性,对于以注重健康的消费者为目标的製造商而言,全面的残留分析在经济上也是可行的。社群媒体和即时资讯共用的兴起提高了消费者对产品成分的审查力度,迫使企业保持严格的检测标准。此外,食品安全事件和召回事件的增加也提高了人们对残留检测的认识,使其成为品质保证计划的重要组成部分。
先进测试技术高成本
资本密集分析设备对小型实验室和食品製造商构成了障碍,尤其是在价格敏感的市场中。先进的 LC-MS/MS 系统需要大量的前期投资和持续的维护成本,这给中型实验室的营运预算带来了压力。现代测试平台的复杂性需要专门的技术知识并增加了人事费用。设备验证和校准的监管合规性要求产生了额外的成本层级,尤其对服务于本地市场的实验室影响更大。然而,技术提供者越来越多地提供租赁模式和共用存取平台,使高级测试功能民主化。这些财务限制导致了市场整合,大型实验室在残留测试领域中占据主导地位。小型企业通常将其测试需求外包给第三方实验室,为测试服务创造了次市场。
細項分析
到2024年,农药将占据44.94%的市场份额,这反映了其在全球食品体系中的广泛使用和全面的监管。重金属仍将是第二大类别,这得益于环境污染问题以及食品中铅、镉和汞监管力度的加强。毒素将成为成长最快的类别,到2030年,其复合年增长率将达到5.08%,这得益于霉菌毒素检测要求的不断提高以及对新型生物毒素检测的需求。 「其他」类别,包括药物、抗生素和化学污染物,将受益于对动物用药品残留和PFAS等新兴污染物的严格审查。
欧盟最新霉菌毒素法规(包括2024年7月生效的2024/1022号法规)是监管因素加速毒素检测需求的一个例子,该法规将设定呕吐基准值(DON)、T-2毒素和HT-2毒素的新最高限量。台湾已对宠物食品中的五种霉菌毒素设定了允许限量,其中包括狗粮中2 ppm的催吐毒素和猫粮中5 ppm的催吐毒素。脂多醣印迹聚合物等先进检测方法可使沙门氏菌检测灵敏度达到10 CFU/mL,无需复杂的样品製备即可快速鑑定病原体。
基于液相层析串联质谱 (LC-MS/MS) 的技术将在 2024 年保持市场领导,市场份额达 35.54%,这得益于监管部门的核准以及其在多种残留类型中经过验证的分析性能。基于高效液相层析 (HPLC) 的方法适用于成本敏感的应用,在这些应用中,高通量筛检比最终灵敏度更重要。气相层析串联质谱 (GC-MS/MS) 平台在挥发性化合物分析方面表现出色,尤其是加工食品中的农药残留。基于电感耦合等离子体质谱 (ICP-MS) 的技术可满足重金属检测需求,并具有优异的微量元素分析准确度。
到2030年,NGS/生物感测器技术将以5.44%的复合年增长率加速发展,这得益于快速检测能力和多重分析潜力,这些潜力将改变实验室工作流程。 FDA对Q20+奈米孔定序用于细菌疫情调查的评估表明,监管机构对即时病原体鑑定的兴趣,这有可能将目前2-4週的测序时间缩短至近乎即时的结果。 CRISPR-Cas系统能够快速、高度特异性且经济高效地检测致病性大肠桿菌,从而突破了传统耗时方法的限制。基于免疫测量的平台将继续用于常规筛检应用,而包括表面增强拉曼散射和电化学感测器在内的「其他」技术将提供可携式检测功能。
区域分析
到 2024 年,欧洲将以 34.78% 的份额保持市场领先地位,这得益于欧盟全面的法规结构,包括更新的《污染物法规 (EU) 2023/915》,该法规规定了各种食品污染物的最高含量。该地区的检测基础设施受益于统一的分析标准和强有力的执行机制,这些机制促进了成员国的系统采用。德国、英国和法国的分析能力处于领先地位,而荷兰和比利时是重要的进口门户,需要广泛的测试通讯协定。根据欧洲食品安全局 (EFSA) 的数据,其 2023 年年度报告显示合规率为 99%,仅 2% 超过最大残留基准值,突显了该地区严格的监控方法。最近的监管更新,包括氟唑菌酰胺、Lambda-Cyhalothrin、Metalaxyl和尼古丁的新最大残留基准值,将于 2025 年 1 月生效,表明安全标准不断完善。
受监管现代化努力和主要经济体粮食生产能力不断扩大的推动,亚太地区正加速成为成长最快的地区,到 2030 年复合年增长率将达到 5.24%。中国将于 2024 年发布 47 项新的国家食品安全标准,其中包括 7 种专用检测方法,标誌着其监管与国际最佳实践的接轨。印度食品安全局 (FSSAI) 重新实施食品标籤标准(将于 2023 年 1 月生效)将加强监督机制,而安捷伦与 ICAR-国家葡萄研究中心的战略伙伴关係将开发针对新兴污染物(包括 PFAS 和极性农药)的先进分析工作流程。日本修订了农药和动物用药品的最大残留基准值,使其与国际标准接轨,而韩国更新的农药抗药性标准和食品标籤要求则加强了消费者保护。随着出口导向经济体实施更严格的检测通讯协定以满足国际市场要求,澳洲和印尼代表着巨大的成长机会。
在 FDA 和 USDA 严格监管机制的支持下,北美透过对国内和进口食品供应进行全面检测,维持了重要的市场份额。 FDA 的 2022 年农药残留监测报告显示,国内样品的合规率为 96.2%,进口样品的合规率为 89.5%,凸显了检测在全球食品贸易中的重要作用。该地区受益于先进的实验室基础设施和技术创新,SGS 等公司透过升级主要製造地的设施来扩展食品检测能力。随着巴西采用新的法规结构和修订的食品接触材料技术法规,南美洲已成为一个成长机会,而阿根廷、哥伦比亚和智利正在加强其出口检测能力以满足国际要求。中东和非洲代表新兴市场,随着食品安全意识的提高和出口需求的扩大,基础设施投资和製定法规结构提供了长期成长潜力。
其他福利:
- Excel 格式的市场预测 (ME) 表
- 3个月的分析师支持
目录
第一章 引言
- 研究假设和市场定义
- 调查范围
第二章调查方法
第三章执行摘要
第四章 市场状况
- 市场概况
- 市场驱动因素
- 世界各地严格的食品安全法规
- 洁净标示食品和饮料的需求激增
- 测试技术的进步
- 食物中毒发生率增加
- 农药在农业上的广泛使用
- 食品召回增加
- 市场限制
- 先进测试技术高成本
- 各地区残留基准值缺乏标准化
- 新兴国家基础建设有限
- 小农和生产者缺乏意识
- 监管状况
- 技术展望
- 五力分析
- 新进入者的威胁
- 买方的议价能力
- 供应商的议价能力
- 替代品的威胁
- 竞争对手之间的竞争
第五章市场规模及成长预测
- 按残留物类型
- 杀虫剂
- 重金属
- 危险物质
- 其他的
- 依技术
- 基于LC-MS/MS
- 基于HPLC
- 基于GC-MS/MS
- 基于ICP-MS
- 基于免疫检测测定
- NGS/生物感测器
- 其他的
- 按用途
- 农作物
- 饲料和宠物食品
- 饮食
- 肉类/家禽
- 乳製品
- 水果和蔬菜
- 加工食品
- 饮料
- 按检验方式
- 实验室考试
- 检测套组
- 按地区
- 北美洲
- 美国
- 加拿大
- 墨西哥
- 北美其他地区
- 欧洲
- 德国
- 英国
- 义大利
- 法国
- 西班牙
- 荷兰
- 其他欧洲国家
- 亚太地区
- 中国
- 印度
- 日本
- 澳洲
- 印尼
- 韩国
- 其他亚太地区
- 南美洲
- 巴西
- 阿根廷
- 南美洲其他地区
- 中东和非洲
- 南非
- 沙乌地阿拉伯
- 阿拉伯聯合大公国
- 其他中东和非洲地区
- 北美洲
第六章 竞争态势
- 市场集中度
- 策略倡议
- 市场排名分析
- 公司简介
- SGS Societe Generale de Surveillance SA
- Eurofins Scientific
- Bureau Veritas
- Intertek Group plc
- Tuv Sud EV(TUV SUD)
- NSF
- Merieux Nutrisciences Corporation
- ALS Global
- AsureQuality
- SCIEX(Danaher)
- Thermo Fisher Scientific
- Hewlett-Packard(Agilent Technologies, Inc.)
- Waters Corporation
- Shimadzu Corporation
- PerkinElmer NV
- Neogen Corporation
- Charm Sciences
- DSM-Firmenich(Romer Labs)
- Labcorp Crop
- Symbio Labs
第七章 市场机会与未来展望
The food residue testing market size is expected to reach USD 2.22 billion in revenue by 2025 and is forecast to expand to USD 2.81 billion by 2030, registering a 4.83% CAGR.

Growth is fuelled by stricter global maximum residue limits, rapid technology upgrades, and rising consumer insistence on verifiable food purity. The European Food Safety Authority's 2023 annual report indicated that 99% of food samples complied with EU regulations, yet 2% exceeded maximum residue levels, underscoring the critical need for robust testing infrastructure . Governments tighten rules faster than most producers can adapt, extending mandatory testing to new residue classes and mandating real-time monitoring across import and domestic supply chains. Convergence of mass-spectrometry, sequencing and biosensor platforms shortens analysis cycles, while the accelerating cadence of food recalls keeps testing demand structurally high. The food residue testing market remains fragmented, creating meaningful scope for consolidation as service providers seek scale advantages in instrumentation investment, data analytics, and geographic reach.
Global Residue Testing Market Trends and Insights
Stringent Global Food Safety Regulations
Regulatory tightening accelerates testing demand as governments implement more restrictive maximum residue limits and expand monitoring scope. The EU's implementation of Regulation 2023/915 established comprehensive contaminant thresholds, while the FDA's updated mycotoxin monitoring program now includes T-2/HT-2 toxins and zearalenone using multi-mycotoxin analytical methods. China's release of 47 new national food safety standards in 2024, including 7 dedicated testing methodologies, signals regulatory convergence toward international best practices. In July 2024 Taiwan's introduction of safety tolerance levels for 5 additional mycotoxins in pet food, including vomitoxin and fumonisin, demonstrates expanding regulatory scope beyond human consumption. The FDA's Laboratory Accreditation for Analyses of Foods (LAAF) program mandate for mycotoxin testing starting December 2024 creates structural demand for accredited testing capabilities. Brazil's adoption of new regulatory frameworks for food products and revised technical regulations for food contact metals further exemplifies global regulatory harmonization driving systematic testing requirements. These regulatory developments across major markets necessitate increased investment in testing infrastructure and analytical capabilities. The harmonization of international standards creates opportunities for testing service providers while ensuring consistent food safety measures across global supply chains.
Surge in Demand for Clean-label Food and Beverage Products
Consumer preference for transparent, minimally processed foods drives manufacturers to implement comprehensive residue testing protocols that verify clean-label claims. This trend particularly impacts premium food segments where brands differentiate through certified purity levels and absence of synthetic residues. The clean-label movement extends beyond pesticides to encompass heavy metals, processing aids, and packaging contaminants, expanding the testing scope per product. Retailers increasingly require third-party verification of clean-label assertions, creating systematic testing demand across supply chains. The premium pricing associated with clean-label products justifies enhanced testing investments, making comprehensive residue analysis economically viable for manufacturers targeting health-conscious consumers. The rise of social media and instant information sharing has amplified consumer scrutiny of product ingredients, forcing companies to maintain rigorous testing standards. Additionally, the growing number of food safety incidents and recalls has heightened awareness about residue testing, making it an essential component of quality assurance programs.
High Cost of Advanced Testing Technologies
Capital-intensive analytical equipment creates barriers for smaller laboratories and food producers, particularly in price-sensitive markets. Advanced LC-MS/MS systems require substantial upfront investments and ongoing maintenance costs that strain operational budgets for mid-tier testing facilities. The complexity of modern testing platforms demands specialized technical expertise, adding personnel costs that compound equipment expenses. Regulatory compliance requirements for instrument validation and calibration create additional cost layers that particularly impact laboratories serving local markets. However, technology providers increasingly offer leasing models and shared-access platforms that democratize advanced testing capabilities. These financial constraints have led to market consolidation, with larger laboratories dominating the residue testing landscape. Small and medium-sized enterprises often resort to outsourcing their testing needs to third-party laboratories, creating a secondary market for testing services.
Other drivers and restraints analyzed in the detailed report include:
- Technological Advancements in Testing Techniques
- Rise in Outbreaks of Foodborne Illnesses
- Limited Infrastructure in Developing Countries
For complete list of drivers and restraints, kindly check the Table Of Contents.
Segment Analysis
Pesticides commanded 44.94% market share in 2024, reflecting widespread agrochemical usage and comprehensive regulatory monitoring across global food systems. Heavy metals represent the second-largest category, driven by environmental contamination concerns and tightening limits for lead, cadmium, and mercury in food products. Toxins emerge as the fastest-growing segment at 5.08% CAGR through 2030, propelled by expanding mycotoxin testing requirements and novel biotoxin detection needs. The "Others" category, encompassing drugs, antibiotics, and chemical contaminants, benefits from increased scrutiny of veterinary drug residues and emerging contaminants like PFAS.
The EU's updated mycotoxin regulations, including Regulation 2024/1022 setting new maximum levels for DON, T-2, and HT-2 toxins effective July 2024, exemplify regulatory drivers accelerating toxin testing demand. Taiwan's introduction of tolerance levels for 5 additional mycotoxins in pet food, including 2 ppm vomitoxin for dog food and 5 ppm for cat food, demonstrates expanding regulatory scope beyond human consumption. Advanced detection methods like lipopolysaccharide-imprinted polymers achieve 10 CFU/mL sensitivity for Salmonella detection, enabling rapid on-site pathogen identification without complex preprocessing
LC-MS/MS-based technology maintains market leadership with 35.54% share in 2024, supported by regulatory acceptance and proven analytical performance across diverse residue types. HPLC-based methods serve cost-sensitive applications where high-throughput screening takes precedence over ultimate sensitivity. GC-MS/MS platforms excel in volatile compound analysis, particularly for pesticide residues in processed foods. ICP-MS-based technology addresses heavy metal detection requirements with exceptional precision for trace elemental analysis.
NGS/biosensor technology accelerates at 5.44% CAGR through 2030, driven by rapid detection capabilities and multiplex analysis potential that transforms laboratory workflows. The FDA's evaluation of Q20+ Nanopore Sequencing for bacterial outbreak investigations demonstrates regulatory interest in real-time pathogen identification, potentially reducing current 2-4 week sequencing timelines to near real-time results. CRISPR-Cas systems enable rapid pathogenic E. coli detection with high specificity and cost-effectiveness, addressing limitations of traditional time-consuming methods. Immunoassay-based platforms continue serving routine screening applications, while "Others" encompass emerging technologies like surface-enhanced Raman scattering and electrochemical sensors that offer portable detection capabilities.
The Residue Testing Market Report is Segmented by Residue Type (Pesticides, Heavy Metals, and More), Technology (HPLC-Based, LC-MS/MS-based, and More), Application (Feed and Pet Food, Agricultural Crops, and Food and Beverage), Testing Mode (Lab Testing and Testing Kits) and Geography (North America, Europe, Asia-Pacific, South America, and Middle East and Africa). The Market Forecasts are Provided in Terms of Value (USD).
Geography Analysis
Europe maintains market leadership with 34.78% share in 2024, anchored by the EU's comprehensive regulatory framework including the updated Contaminants Regulation 2023/915 that establishes maximum levels for various food contaminants. The region's testing infrastructure benefits from harmonized analytical standards and robust enforcement mechanisms that drive systematic adoption across member states. Germany, United Kingdom, and France lead in analytical capabilities, while Netherlands and Belgium serve as critical import gateways requiring extensive testing protocols. The EFSA's 2023 annual report indicating 99% regulatory compliance yet 2% exceedance of maximum residue levels underscores the region's rigorous monitoring approach according to European Food Safety Authority. Recent regulatory updates include new maximum residue levels for fluxapyroxad, lambda-cyhalothrin, metalaxyl, and nicotine effective January 2025, demonstrating ongoing refinement of safety standards.
Asia-Pacific accelerates as the fastest-growing region at 5.24% CAGR through 2030, driven by regulatory modernization initiatives and expanding food production capacity across major economies. China's release of 47 new national food safety standards in 2024, including 7 dedicated testing methodologies, signals regulatory convergence toward international best practices. India's FSSAI re-operationalization of food labeling standards effective January 2023 strengthens oversight mechanisms, while Agilent's strategic partnership with ICAR-National Research Centre for Grapes develops advanced analytical workflows for emerging contaminants including PFAS and polar pesticides. Japan's revision of maximum residue limits for pesticides and veterinary drugs aligns with international standards, while South Korea's updates to pesticide tolerance standards and food labeling requirements enhance consumer protection. Australia and Indonesia represent significant growth opportunities as export-oriented economies implement stricter testing protocols to meet international market requirements.
North America maintains substantial market presence supported by stringent FDA and USDA oversight mechanisms that drive comprehensive testing across domestic and imported food supplies. The FDA's FY 2022 Pesticide Residue Monitoring Report revealed 96.2% compliance for domestic samples versus 89.5% for imports, highlighting the critical role of testing in global food trade. The region benefits from advanced laboratory infrastructure and technological innovation, with companies like SGS expanding food testing capacity through facility upgrades in key manufacturing hubs . South America emerges as a growth opportunity driven by Brazil's adoption of new regulatory frameworks and revised technical regulations for food contact materials, while Argentina, Colombia, and Chile enhance export testing capabilities to meet international requirements. Middle East and Africa represent developing markets where infrastructure investments and regulatory framework development create long-term growth potential as food safety awareness increases and export ambitions expand.
- SGS Societe Generale de Surveillance SA
- Eurofins Scientific
- Bureau Veritas
- Intertek Group plc
- Tuv Sud E.V. (TUV SUD)
- NSF
- Merieux Nutrisciences Corporation
- ALS Global
- AsureQuality
- SCIEX (Danaher)
- Thermo Fisher Scientific
- Hewlett-Packard (Agilent Technologies, Inc.)
- Waters Corporation
- Shimadzu Corporation
- PerkinElmer NV
- Neogen Corporation
- Charm Sciences
- DSM-Firmenich (Romer Labs)
- Labcorp Crop
- Symbio Labs
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 Introduction
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 Research Methodology
3 Executive Summary
4 Market Landscape
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Stringent Global Food Safety Regulations
- 4.2.2 Surge in Demand for Clean-label Food and Beverage Products
- 4.2.3 Technological Advancements in Testing Techniques
- 4.2.4 Rise in Outbreaks of Foodborne Illnesses
- 4.2.5 Widespread Use of Agrochemicals in Farming
- 4.2.6 Increased Incidence of Food Recalls
- 4.3 Market Restraints
- 4.3.1 High Cost of Advanced Testing Technologies
- 4.3.2 Lack of Standardization in Residue Limits Across Regions
- 4.3.3 Limited Infrastructure in Developing Countries
- 4.3.4 Lack of Awareness Among Small-Scale Farmers and Producers
- 4.4 Regulatory Landscape
- 4.5 Technological Outlook
- 4.6 Porter's Five Forces
- 4.6.1 Threat of New Entrants
- 4.6.2 Bargaining Power of Buyers
- 4.6.3 Bargaining Power of Suppliers
- 4.6.4 Threat of Substitutes
- 4.6.5 Competitive Rivalry
5 Market Size and Growth Forecasts (Value)
- 5.1 By Residue Type
- 5.1.1 Pesticides
- 5.1.2 Heavy Metals
- 5.1.3 Toxins
- 5.1.4 Others
- 5.2 By Technology
- 5.2.1 LC-MS/MS-based
- 5.2.2 HPLC-based
- 5.2.3 GC-MS/MS-based
- 5.2.4 ICP-MS-based
- 5.2.5 Immunoassay-based
- 5.2.6 NGS/Biosensor
- 5.2.7 Others
- 5.3 By Application
- 5.3.1 Agricultural Crops
- 5.3.2 Feed and Pet Food
- 5.3.3 Food and Beverage
- 5.3.3.1 Meat and Poultry
- 5.3.3.2 Dairy
- 5.3.3.3 Fruits and Vegetables
- 5.3.3.4 Processed Food
- 5.3.3.5 Beverages
- 5.4 By Testing Mode
- 5.4.1 Lab Testing
- 5.4.2 Testing Kits
- 5.5 By Geography
- 5.5.1 North America
- 5.5.1.1 United States
- 5.5.1.2 Canada
- 5.5.1.3 Mexico
- 5.5.1.4 Rest of North America
- 5.5.2 Europe
- 5.5.2.1 Germany
- 5.5.2.2 United Kingdom
- 5.5.2.3 Italy
- 5.5.2.4 France
- 5.5.2.5 Spain
- 5.5.2.6 Netherlands
- 5.5.2.7 Rest of Europe
- 5.5.3 Asia-Pacific
- 5.5.3.1 China
- 5.5.3.2 India
- 5.5.3.3 Japan
- 5.5.3.4 Australia
- 5.5.3.5 Indonesia
- 5.5.3.6 South Korea
- 5.5.3.7 Rest of Asia-Pacific
- 5.5.4 South America
- 5.5.4.1 Brazil
- 5.5.4.2 Argentina
- 5.5.4.3 Rest of South America
- 5.5.5 Middle East and Africa
- 5.5.5.1 South Africa
- 5.5.5.2 Saudi Arabia
- 5.5.5.3 United Arab Emirates
- 5.5.5.4 Rest of Middle East and Africa
- 5.5.1 North America
6 Competitive Landscape
- 6.1 Market Concentration
- 6.2 Strategic Moves
- 6.3 Market Ranking Analysis
- 6.4 Company Profiles (includes Global level Overview, Market level overview, Core Segments, Financials (if available), Strategic Information, Market Rank/Share, Products and Services, Recent Developments)
- 6.4.1 SGS Societe Generale de Surveillance SA
- 6.4.2 Eurofins Scientific
- 6.4.3 Bureau Veritas
- 6.4.4 Intertek Group plc
- 6.4.5 Tuv Sud E.V. (TUV SUD)
- 6.4.6 NSF
- 6.4.7 Merieux Nutrisciences Corporation
- 6.4.8 ALS Global
- 6.4.9 AsureQuality
- 6.4.10 SCIEX (Danaher)
- 6.4.11 Thermo Fisher Scientific
- 6.4.12 Hewlett-Packard (Agilent Technologies, Inc.)
- 6.4.13 Waters Corporation
- 6.4.14 Shimadzu Corporation
- 6.4.15 PerkinElmer NV
- 6.4.16 Neogen Corporation
- 6.4.17 Charm Sciences
- 6.4.18 DSM-Firmenich (Romer Labs)
- 6.4.19 Labcorp Crop
- 6.4.20 Symbio Labs








