 |
市场调查报告书
商品编码
1846201
水杨酸:市场占有率分析、产业趋势、统计数据、成长预测(2025-2030)Salicylic Acid - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030) |
||||||
※ 本网页内容可能与最新版本有所差异。详细情况请与我们联繫。
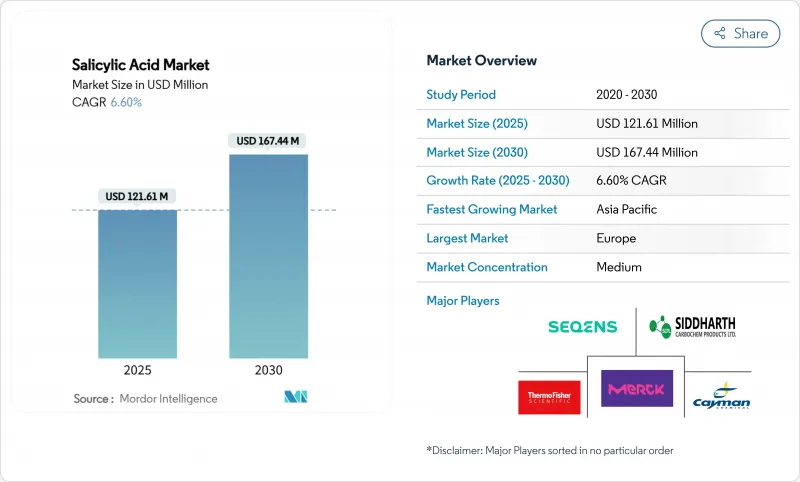
预计到 2025 年,水杨酸市值将达到 1.2161 亿美元,到 2030 年将成长至 1.6744 亿美元,复合年增长率为 6.60%。
市场成长主要受治疗核准、洁净标示防腐剂需求以及农业应用等因素驱动。製药业需要水杨酸,因为它具有角质溶解和抗炎特性;个人护理产品製造商则将其用于痤疮治疗和去角质产品。此外,美国和欧洲监管机构提高了儿童产品的安全要求,但放宽了食品和饮料应用的耐受性。一种基于二氧化碳的合成方法,产率高达92.68%,提高了生产效率并减少了排放。此外,其粉末/晶体形式具有高度稳定性,使其在製药和个人保健产品应用领域占据市场领先地位。这种晶体形式对于痤疮霜、去头皮屑洗髮精和药用贴片等局部治疗产品至关重要。在新兴市场完善的法律规范的支持下,製药业持续成长,这主要得益于水杨酸在非处方药和处方药中的应用。
全球水杨酸市场趋势与洞察
製药业对水杨酸的需求不断增长
药物应用将透过拓展治疗适应症和改进製剂技术来推动市场扩张。先进的递送系统,特别是超分子製剂,与传统製剂相比,已展现出更显着的胶原蛋白密度提升和更低的皮肤刺激性,从而克服了以往限制其在更广泛治疗中应用的局限性。药物应用领域的专利活动仍然活跃,近期申请主要集中于联合治疗和新型递送机制,这些机制旨在提高生物有效性并最大限度地减少胃肠道副作用。製药业正受益于完善的监管途径以及人们对消化器官系统在传统皮肤科用途之外的抗炎特性的日益认可。此外,该化合物已确立的安全性以及监管部门的核准也鼓励生产商将其添加到各种製剂中。河北精业集团和山东新华公司等主要供应商在全球药用级水杨酸的生产中发挥关键作用。最后,受消费者自我护理偏好的推动,含有水杨酸的非处方药数量迅速增长,也显着促进了市场成长。
对护肤和个人保健产品的需求不断增长
消费者对经科学检验的活性成分的偏好持续推动着水杨酸在化妆品配方中的应用。然而,日益严格的安全监管正在影响这种成分的使用方式。例如,美国委员会消费者安全科学委员会核准将儿童化妆品中水杨酸的浓度限制在0.1%以内(用于经皮给药)。在美国,监管力道也随之加大,美国食品药物管理局(FDA)曾向Skin Beauty Solutions公司发出警告信,指出其未经批准的化学换肤产品含有高浓度水杨酸。这促使生产商更加重视开发合规且安全的配方。此外,《化妆品监管现代化法案》实施了新的註册要求,提高了合规标准和市场准入通讯协定。化妆品和个人护理产品生产商正在使用水杨酸,以满足市场对经科学检验的护肤产品的需求。艾伯维旗下子公司艾尔建美学于 2024 年 4 月推出了两款 SkinMedica 产品,用于治疗痤疮。痤疮净肤护理和毛孔净化洁面啫咖哩均采用 2% 的包封水杨酸,可实现控释并减少刺激。
替代产品的可用性
在医药和化妆品领域,替代活性成分的出现带来了竞争压力,尤其是在对成本敏感的细分市场,因为功效上的差异可能不足以支撑高价。 β-羟基酸(如水杨酸甜菜碱)和α-羟基酸(如乙醇酸)具有类似的去角质功效,同时可能具有低致敏性,因此对那些对水杨酸有副作用的敏感肌肤消费者俱有吸引力。在农业应用领域,合成植物生长调节剂和天然化合物都在争夺市场份额,但水杨酸作为生长促进剂和抗病性增强剂的双重作用使其具有差异化优势。含有多种活性成分的混合产品的出现可以减少对水杨酸作为单一成分的依赖,尤其是在化妆品配方中,多功能成分往往占据高端市场。关键配方技术的专利到期可能会加速学名药的竞争和替代成分的推广。市场动态有利于那些具有更高功效/刺激性比的产品,这推动了水杨酸配方和竞争性替代成分的创新。
细分市场分析
凭藉成熟的生产流程和经济高效的生产模式,粉末/晶体製剂将继续保持市场领先地位,到2024年市场份额将达到71.78%。液体製剂将呈现加速成长,年复合成长率将达到7.44%,因为製造商持续致力于改善其生物有效性和给药便利性。传统粉末製剂受益于其稳定性优势和完善的供应链基础设施,尤其是在对剂量精准和保质期要求较高的製药应用领域。
由于其优异的溶解性、高效的皮肤吸收性和便捷的使用方式,水杨酸市场对液态製剂的需求日益增长。这些特性满足了市场对即用型化妆品的需求,尤其是针对痤疮治疗、去角质和清洁化妆水等产品。将水杨酸配製成稳定的液体基质可以增强其功效。此外,新型给药系统,例如经皮吸收贴片和控制释放凝胶,结合了固态製剂的稳定性和液体製剂的快速起效性。这些进步正在推动市场对能够提供最佳功效、最大限度减少皮肤刺激并提供便捷使用方法的水杨酸产品的需求。
全球水杨酸市场分析按应用领域(医药、化妆品及个人护理、食品饮料及其他)、形态(粉末/晶体和液体)以及地区(北美、欧洲、亚太、南美以及中东和非洲)进行细分。市场预测以美元计价。
区域分析
凭藉完善的药品生产基础设施和严格的法规结构(检验产品品质和安全标准),欧洲将在2024年继续保持市场主导,市场份额将达到31.17%。欧洲药品管理局和各国监管机构对欧洲市场进行全面监管,增强了消费者对含水杨酸产品的信心,同时也为生产商提供了明确的合规路径。欧洲生产商利用先进的生产技术和成熟的分销网络,服务国内和出口市场,尤其在高价值的药品和化妆品应用领域具有优势。
亚太地区预计将成为成长最快的地区,到2030年复合年增长率将达到9.83%,这主要得益于不断扩大的製药产能、日益增长的农业应用以及消费者对个人保健产品中活性成分益处的认知不断提高。中国正透过对製造业的大量投资引领区域成长,包括建造符合环保要求并采用先进製程技术的新生产设施。此外,北美市场已趋于成熟,对药品、化妆品和新兴食品保鲜应用的需求稳定,这得益于完善的法律规范以及消费者对活性成分益处的认可。美国食品药物管理局(FDA)就水杨酸在各种应用中的使用提供了明确的指导,为产品开发和打入市场策略提供了监管确定性。
南美和中东及非洲(MEA)市场为水杨酸提供了成长机会,这主要得益于都市化、可支配收入的增加以及对药品和个人保健产品需求的增长。巴西和墨西哥的国内药品生产和非处方护肤正在蓬勃发展,推动了水杨酸製剂的广泛应用。在中东及非洲地区,消费者对个人卫生和皮肤健康的日益重视,也带动了痤疮治疗和去角质产品中对水杨酸的需求。然而,市场成长也面临许多限制因素,包括产能有限、地区法规不尽相同以及对进口的依赖。全球製造商正致力于建立策略伙伴关係和在地化布局,以扩大市场覆盖范围,并在这些地区建立更强大的供应链。
其他福利:
- Excel格式的市场预测(ME)表
- 3个月的分析师支持
目录
第一章 引言
- 研究假设和市场定义
- 调查范围
第二章调查方法
第三章执行摘要
第四章 市场情势
- 市场概览
- 市场驱动因素
- 製药业对水杨酸的需求不断增长
- 对护肤和个人保健产品的需求不断增长
- 洁净标示饮料储存认证数量增加
- 包装食品产业对水杨酸作为食品防腐剂的需求不断增长
- 对植物生长调节剂的需求日益增长
- 消费者越来越偏好选择经过皮肤科测试的天然成分。
- 市场限制
- 替代产品的供应情况
- 原物料价格波动
- 严格的监管核准和合规要求
- 潜在健康风险和皮肤敏感性
- 供应链分析
- 监理展望
- 波特五力分析
- 新进入者的威胁
- 买方的议价能力
- 供应商的议价能力
- 替代品的威胁
- 竞争对手之间的竞争
第五章 市场规模与成长预测
- 透过使用
- 製药
- 化妆品和个人护理
- 饮食
- 其他的
- 按形式
- 粉末/晶体
- 液体
- 按地区
- 北美洲
- 美国
- 加拿大
- 墨西哥
- 北美其他地区
- 欧洲
- 德国
- 法国
- 英国
- 西班牙
- 义大利
- 俄罗斯
- 其他欧洲国家
- 亚太地区
- 中国
- 印度
- 日本
- 澳洲
- 其他亚太地区
- 南美洲
- 巴西
- 阿根廷
- 其他南美
- 中东和非洲
- 南非
- 沙乌地阿拉伯
- 其他中东和非洲地区
- 北美洲
第六章 竞争情势
- 市场集中度
- 策略倡议
- 市场排名分析
- 公司简介
- Seqens Group
- Merck KGaA
- Thermo Fisher Scientific
- Cayman Chemical Company
- Siddharth Carbochem Products
- Lona Industries(Alta Laboratories)
- SimSon Pharma Limited
- Hebei Jingye Group
- Zhenjiang Gaopeng Pharmaceutical
- Spectrum Chemical Mfg
- Avantor Inc.
- Emco Group(Emco Dyestuff Pvt Ltd)
- Alpha Chemika
- Flychem Private Limited
- Central Drug House
- MedChemExpress
- The Andhra Sugars Limited.
- GFS Chemicals
- Plater Group
- Honeywell Research Chemical
第七章 市场机会与未来展望
The salicylic acid market is estimated to be USD 121.61 million in 2025 and is expected to grow to USD 167.44 million by 2030, at a CAGR of 6.60%.

This market growth stems from therapeutic approvals, clean-label preservative demand, and agricultural applications. The pharmaceutical industry requires salicylic acid for its keratolytic and anti-inflammatory properties, while personal care manufacturers use it in acne treatment and exfoliation products. Besides, the United States and European regulators have increased safety requirements for children's products but expanded allowances in food and beverage applications. CO2-based synthesis, achieving 92.68% yield, has improved production efficiency and reduced emissions. Moreover, the powder/crystal form leads the market due to its stability and applications in pharmaceutical and personal care products. This crystalline form is essential in topical treatments, including acne creams, anti-dandruff shampoos, and medicated patches. The pharmaceutical segment maintains growth through salicylic acid's use in over-the-counter and prescription products, supported by established regulatory frameworks in developed markets.
Global Salicylic Acid Market Trends and Insights
Increasing Demand for Salicylic Acid in the Pharmaceutical Industry
Pharmaceutical applications drive market expansion through expanding therapeutic indications and enhanced formulation technologies. Advanced delivery systems, particularly supramolecular formulations, demonstrate superior collagen density improvement and reduced skin irritation compared to conventional preparations, addressing historical limitations that constrained broader therapeutic adoption. Patent activity in pharmaceutical applications remains robust, with recent filings focusing on combination therapies and novel delivery mechanisms that enhance bioavailability while minimizing gastrointestinal side effects. The pharmaceutical segment benefits from established regulatory pathways and growing recognition of salicylic acid's anti-inflammatory properties beyond traditional dermatological applications. Moreover, the compound's established safety profile and regulatory approvals encourage manufacturers to incorporate it into various formulations. Key suppliers such as Hebei Jingye Group, Shandong Xinhua Company, and others play a significant role in producing pharmaceutical-grade salicylic acid for global markets. Finally, the surge in over-the-counter pharmaceutical products containing salicylic acid, driven by consumer preference for self-care solutions, also contributes significantly to the market's growth.
Rising Demand in Skincare and Personal Care Products
Consumer preference for scientifically validated active ingredients continues to drive the adoption of salicylic acid in cosmetic formulations. However, increasing regulatory scrutiny around safety is shaping how the ingredient is used. For instance, the European Commission's Scientific Committee on Consumer Safety recently limited the concentration of salicylic acid in children's cosmetic products to 0.1% for dermal applications, reflecting growing safety concerns while still allowing higher concentrations in adult products . In the United States, regulatory enforcement is also tightening, as evidenced by the United States Food and Drug Administration (FDA)'s warning letter to Skin Beauty Solutions regarding unapproved chemical peel products containing high concentrations of salicylic acid. This has prompted manufacturers to focus on developing compliant, safe formulations. Additionally, the Modernization of Cosmetics Regulation Act implements new registration requirements, increasing compliance standards and market entry protocols. Cosmetic and personal care manufacturers are integrating salicylic acid into formulations to address market demand for scientifically validated skincare products. Allergan Aesthetics, an AbbVie subsidiary, launched two SkinMedica products in April 2024 for acne treatment. The Acne Clarifying Treatment and Pore Purifying Gel Cleanser utilize 2% encapsulated salicylic acid for controlled release and reduced irritation.
Availability of Substitutes
Alternative active ingredients in pharmaceutical and cosmetic applications create competitive pressure, particularly in cost-sensitive market segments where efficacy differences may not justify premium pricing. Beta-hydroxy acids like betaine salicylate and alpha-hydroxy acids such as glycolic acid offer similar exfoliation benefits with potentially reduced irritation profiles, appealing to sensitive-skin consumers who experience adverse reactions to salicylic acid. In agricultural applications, synthetic plant growth regulators and alternative natural compounds compete for market share, though salicylic acid's dual role as both growth promoter and a disease resistance enhancer provides differentiation advantages. The availability of combination products that blend multiple active ingredients can reduce reliance on salicylic acid as a standalone component, particularly in cosmetic formulations where multi-functional ingredients command premium positioning. Patent expiration on key formulation technologies may accelerate generic competition and substitute adoption. Market dynamics favor products that demonstrate superior efficacy-to-irritation ratios, driving innovation in both salicylic acid formulations and competitive alternatives.
Other drivers and restraints analyzed in the detailed report include:
- Rise in Clean-Label Beverage Preservation Approvals
- Increasing Demand for Salicylic Acid in the Packaged Food Sector as a Food Preservative
- Price Volatility of Raw Materials
For complete list of drivers and restraints, kindly check the Table Of Contents.
Segment Analysis
Powder/crystal forms maintain market leadership with a 71.78% share in 2024, supported by established manufacturing processes and cost-effective production economics. Liquid formulations exhibit accelerated growth at 7.44% CAGR as manufacturers develop enhanced bioavailability and application convenience. Traditional powder forms benefit from stability advantages and established supply chain infrastructure, particularly in pharmaceutical applications where precise dosing and extended shelf life remain critical requirements.
The salicylic acid market demonstrates increased adoption of liquid formulations, attributed to their superior solubility, efficient skin absorption, and application convenience. These characteristics address market demand for ready-to-use cosmetic products, specifically in acne treatments, exfoliators, and clarifying toners. The efficacy of salicylic acid increases when formulated in stable liquid bases. Furthermore, emerging delivery systems, including transdermal patches and controlled-release gels, integrate the advantages of solid formulations' stability with liquid forms' rapid effectiveness. These advancements drive market demand for salicylic acid products that deliver optimal performance, minimize skin sensitivity, and provide efficient application methods.
The Global Salicylic Acid Market Analysis is Segmented by Application (Pharmaceuticals, Cosmetics and Personal Care, Food and Beverages, and Others), Form (Powder/Crystal and Liquid), and Geography (North America, Europe, Asia-Pacific, South America, and Middle East and Africa). The Market Forecasts are Provided in Terms of Value (USD).
Geography Analysis
Europe maintains market leadership with a 31.17% share in 2024, supported by established pharmaceutical manufacturing infrastructure and stringent regulatory frameworks that validate product quality and safety standards. The region benefits from comprehensive regulatory oversight through the European Medicines Agency and national authorities, creating consumer confidence in salicylic acid-containing products while establishing clear compliance pathways for manufacturers. European manufacturers leverage advanced production technologies and established distribution networks to serve both domestic and export markets, with particular strength in high-value pharmaceutical and cosmetic applications.
The Asia-Pacific region emerges as the fastest-growing region, with a 9.83% CAGR through 2030, driven by expanding pharmaceutical manufacturing capacity, increasing agricultural applications, and rising consumer awareness of the benefits of active ingredients in personal care products. China leads regional growth through significant investments in manufacturing, including the establishment of new production facilities that incorporate environmental compliance measures and advanced process technologies. Moreover, North America represents a mature market with stable demand patterns across pharmaceutical, cosmetic, and emerging food preservation applications, supported by comprehensive regulatory frameworks and established consumer acceptance of active ingredient benefits. The United States Food and Drug Administration (FDA)'s clear guidance on salicylic acid use in various applications provides regulatory certainty that supports product development and market entry strategies.
South America, and Middle East and Africa (MEA) markets present growth opportunities for salicylic acid, driven by urbanization, higher disposable incomes, and increased demand for pharmaceutical and personal care products. Brazil and Mexico are experiencing growth in their domestic pharmaceutical manufacturing and over-the-counter skincare segments, leading to increased adoption of salicylic acid formulations. The Middle East and Africa (MEA) region shows increased consumer awareness of personal hygiene and skin health, resulting in higher demand for salicylic acid in acne treatments and exfoliating products. However, market growth faces constraints from limited manufacturing capabilities, varying regulations across regions, and dependence on imports. Global manufacturers are focusing on strategic partnerships and local presence to expand their market reach and establish stronger supply chains in these regions.
- Seqens Group
- Merck KGaA
- Thermo Fisher Scientific
- Cayman Chemical Company
- Siddharth Carbochem Products
- Lona Industries (Alta Laboratories)
- SimSon Pharma Limited
- Hebei Jingye Group
- Zhenjiang Gaopeng Pharmaceutical
- Spectrum Chemical Mfg
- Avantor Inc.
- Emco Group (Emco Dyestuff Pvt Ltd)
- Alpha Chemika
- Flychem Private Limited
- Central Drug House
- MedChemExpress
- The Andhra Sugars Limited.
- GFS Chemicals
- Plater Group
- Honeywell Research Chemical
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET LANDSCAPE
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Increasing Demand for Salicylic Acid in the Pharmaceutical Industry
- 4.2.2 Rising Demand in Skincare and Personal Care Products
- 4.2.3 Rise in Clean-Label Beverage Preservation Approvals
- 4.2.4 Increasing Demand for Salicylic Acid in the Packaged Food Sector as a Food Preservative
- 4.2.5 Growing Need for Plant Growth Regulators
- 4.2.6 Increasing Consumer Preference for Dermatologically Tested and Natural Ingredients
- 4.3 Market Restraints
- 4.3.1 Availability of Substitutes
- 4.3.2 Price Volatality of Raw materials
- 4.3.3 Stringent Regulatory Approvals and Compliance Requirements
- 4.3.4 Potential Health Risks and Skin Sensitivities
- 4.4 Supply-Chain Analysis
- 4.5 Regulatory Outlook
- 4.6 Porter's Five Forces Analysis
- 4.6.1 Threat of New Entrants
- 4.6.2 Bargaining Power of Buyers
- 4.6.3 Bargaining Power of Suppliers
- 4.6.4 Threat of Substitutes
- 4.6.5 Competitive Rivalry
5 MARKET SIZE AND GROWTH FORECASTS (VALUE, USD MN)
- 5.1 By Application
- 5.1.1 Pharmaceuticals
- 5.1.2 Cosmetics and Personal Care
- 5.1.3 Food and Beverages
- 5.1.4 Others
- 5.2 By Form
- 5.2.1 Powder/Crystal
- 5.2.2 Liquid
- 5.3 By Geography
- 5.3.1 North America
- 5.3.1.1 United States
- 5.3.1.2 Canada
- 5.3.1.3 Mexico
- 5.3.1.4 Rest of North America
- 5.3.2 Europe
- 5.3.2.1 Germany
- 5.3.2.2 France
- 5.3.2.3 United Kingdom
- 5.3.2.4 Spain
- 5.3.2.5 Italy
- 5.3.2.6 Russia
- 5.3.2.7 Rest of Europe
- 5.3.3 Asia-Pacific
- 5.3.3.1 China
- 5.3.3.2 India
- 5.3.3.3 Japan
- 5.3.3.4 Australia
- 5.3.3.5 Rest of Asia-Pacific
- 5.3.4 South America
- 5.3.4.1 Brazil
- 5.3.4.2 Argentina
- 5.3.4.3 Rest of South America
- 5.3.5 Middle East and Africa
- 5.3.5.1 South Africa
- 5.3.5.2 Saudi Arabia
- 5.3.5.3 Rest of Middle East and Africa
- 5.3.1 North America
6 COMPETITIVE LANDSCAPE
- 6.1 Market Concentration
- 6.2 Strategic Moves
- 6.3 Market Ranking Analysis
- 6.4 Company Profiles (includes Global level Overview, Market level overview, Core Segments, Financials as available, Strategic Information, Market Rank/Share for key companies, Products and Services, and Recent Developments)
- 6.4.1 Seqens Group
- 6.4.2 Merck KGaA
- 6.4.3 Thermo Fisher Scientific
- 6.4.4 Cayman Chemical Company
- 6.4.5 Siddharth Carbochem Products
- 6.4.6 Lona Industries (Alta Laboratories)
- 6.4.7 SimSon Pharma Limited
- 6.4.8 Hebei Jingye Group
- 6.4.9 Zhenjiang Gaopeng Pharmaceutical
- 6.4.10 Spectrum Chemical Mfg
- 6.4.11 Avantor Inc.
- 6.4.12 Emco Group (Emco Dyestuff Pvt Ltd)
- 6.4.13 Alpha Chemika
- 6.4.14 Flychem Private Limited
- 6.4.15 Central Drug House
- 6.4.16 MedChemExpress
- 6.4.17 The Andhra Sugars Limited.
- 6.4.18 GFS Chemicals
- 6.4.19 Plater Group
- 6.4.20 Honeywell Research Chemical









