 |
市场调查报告书
商品编码
1852091
货物追踪解决方案:市场份额分析、行业趋势、统计数据和成长预测(2025-2030 年)Track And Trace Solutions - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030) |
||||||
※ 本网页内容可能与最新版本有所差异。详细情况请与我们联繫。
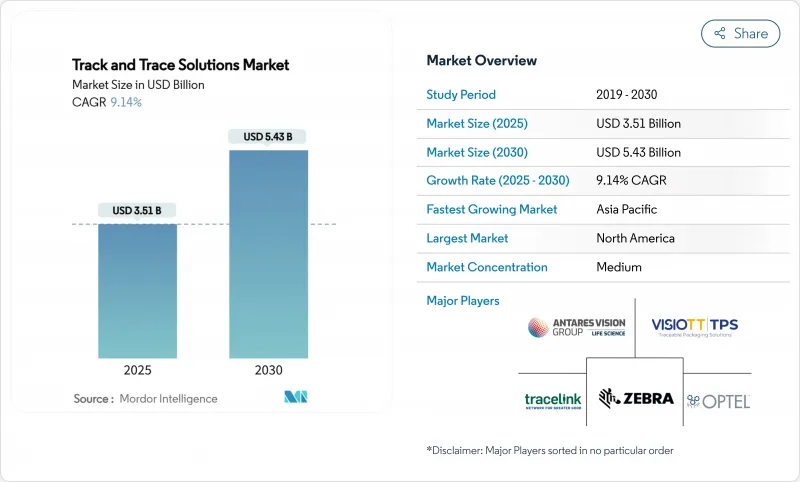
预计到 2025 年,货物追踪解决方案市场价值将达到 35.1 亿美元,到 2030 年将达到 54.3 亿美元,复合年增长率为 9.14%。
药品供应链监管压力日益增大,正将序列化从合规挑战转变为策略差异化因素,促使企业投资更复杂、数据更丰富的平台。随着序列化和聚合向云端架构融合,供应商在探索资料货币化新途径的同时,也面临新的网路安全风险。同时,规模较小的货物追踪解决方案供应商正在新兴地区扩大市场份额,模组化云端架构使他们无需建立全球销售网路也能参与竞争。
全球货物追踪解决方案市场趋势与洞察
全球药品可追溯性监理趋同
标准协调的趋势日益增强,迫使企业设计能够透过一次部署满足多个司法管辖区要求的系统。因此,供应商正大力投资于灵活的资料模型,将国家代码映射到通用核心,从而悄悄降低跨国工厂的生命週期整合成本。一个显着的成果是,製造商开始发布全球提案,而非区域性竞标,这集中了采购权并加速了供应商整合。这种监管趋同还有助于缩短部署週期、加快投资回收期,并释放预算用于进阶分析,因为配置取代了自订编码。
假药威胁日益加剧,凸显了保障病人安全的重要性。
随着造假网路日益复杂,品牌拥有者不得不超越简单的序列化,采用多层安全防护措施,结合防篡改包装、认证演算法和即时认证入口网站。透过在层级构造包装中嵌入唯一标识符,企业可以阻碍力防止未经授权的重新包装,并在发生事故时最大限度地减少召回范围。医疗机构将打击仿冒品产品与提高治疗依从性联繫起来,这推动了政府对更严格的序列化审核的支持。因此,解决方案供应商报告称,市场对行动测试应用程式的需求不断增长,这些应用程式允许现场工作人员每次都检验程式码。
各国时间表的差异会造成投资不确定性。
监管时间表的不统一迫使企业谨慎分配资金,为了保障收入来源,企业往往优先考虑出口市场而非国内市场。财务团队越来越多地采用实物选择权分析来评估产品序列化计划,将区域推广视为一系列顺序选择权,而非确定性计划。解决方案供应商则透过提供模组化许可来应对这项挑战,这种许可仅在各国截止日期临近时才激活,这种定价创新缩短了销售週期。
细分市场分析
至2024年,软体将占货运追踪解决方案市场规模的52.64%,预计到2030年将以8.63%的复合年增长率成长。这种加速成长反映出企业正在从序列化资料中挖掘价值,寻求营运洞察而非仅仅记录合规资讯。平台供应商越来越多地将专业服务捆绑销售,以弥补技能差距,并提高中型製造商的转换成本。值得注意的是,成功的实施往往始于资料管治研讨会,这显示製药公司中IT和品质职能正在融合。
儘管硬体目前仍能满足初步合规需求,但到2030年资本週期完成后,其在追踪解决方案领域的市占率预计将超过29.77%。列印、影像检查和防篡改模组仍然至关重要,供应商正透过模组化设计来减少改装期间的生产线停机时间,从而实现差异化竞争。在硬体领域,RFID印表机和印表机上检验的需求成长最快,反映出包装生产线上感测器的逐步普及。同时,第二代印表机的二手市场流动性增强,降低了新兴国家后入者的进入门槛。
到2024年,条码将占据货物追踪解决方案市场55.76%的份额,但RFID解决方案预计将稳步缩小差距,到2030年将以9.90%的复合年增长率成长。製药仓库为了减少人工扫描,目前正在试行混合标籤方案,即为高价值生技药品配备RFID标籤,而为学名药保留二维条码,这种混合方案兼顾了成本和产能。 RFID托盘的即时位置资料会输入到仓库管理系统(WMS)演算法中,从而缩短拣货时间,这一优势在大型配销中心悄悄抵销了标籤的成本。
条码技术的韧性,得益于扫描器的普及和监管机构的持续认可,将确保该技术在可预见的未来保持其相关性。然而,被动式超高频(UHF)嵌体价格的下降表明,无线射频识别(RFID)技术的广泛应用即将迎来曲折点。多个国家医疗服务机构对医院物流中RFID的需求日益增长,这便是例证,并在供应链中产生了拉动效应。这种日益增长的偏好正促使包装生产线原始设备製造商(OEM)将RFID通道整合到标准设备中。
区域分析
北美仍将是最大的区域市场,预计到2024年将占据货物追踪解决方案市场42.24%的份额。这主要得益于《药品供应链安全法案》(Drug Supply Chain Security Act)的要求,该法案规定到2025年8月必须实现药品单元层级的可追溯性。因此,即使是小型学名药公司也在加快计划以避免供应中断,从而推动了对检验服务的短期需求。该地区成熟的电子健康记录基础设施能够将序列化数据与临床结果关联起来,形成反馈迴路,为基于价值的合约提供资讯。这种关联也促使保险公司增加对分析技术的投资,以检验病患在接受特殊治疗时的依从性。大型批发商正在将序列化技术与机器人技术结合,以减少人工病例处理,并重新调整物流中心的劳动力分配。
亚太地区将呈现最快的成长轨迹,预计复合年增长率将达到10.29%,这主要得益于中国强有力的仿冒品宣传活动和印度的出口激励政策。各国政府通常会将在地化内容法规与可追溯性津贴结合,鼓励国内IT企业与全球解决方案提供者合作。跨国製造商选择可设定的云端平台,以便在规则成熟后,在紧迫的时间节点内切换特定国家的模组。同样的架构也支援语言在地化,这对于语言多样化的地区而言,是一个微妙但至关重要的成功因素。电子商务的快速发展正迫使监管机构加强小包裹层级的检验,进一步加速了可追溯性技术的普及。
欧洲依然是关键所在,随着《反假药指令》的日趋完善,药品检验已下放到药局柜檯。各国药品检验机构的资料库已投入运行,支援涵盖几乎所有配药点的密集扫描网路。製造商正透过在纸盒中嵌入防篡改封条来应对这项挑战,这项设计选择提高了全球消费者的期望。环境永续性是该地区的下一个关注点,相关人员在探索如何利用序列化数据来支持碳足迹报告,这展现了追踪基础设施的多方面潜力。
其他好处
- Excel格式的市场预测(ME)表
- 3个月的分析师支持
目录
第一章 引言
- 研究假设和市场定义
- 调查范围
第二章调查方法
第三章执行摘要
第四章 市场情势
- 市场概览
- 市场驱动因素
- 全球药品可追溯性法规的趋同(世卫组织、国际标准化组织)
- 假药威胁日益加剧:保障病人安全刻不容缓。
- 直接面向患者和电子商务管道的激增需要端到端的可视性。
- 医药供应链数位化及云端原生SaaS的采用
- 转向个人化、低剂量治疗和灵活的治疗连续性是必要的。
- 品牌声誉和避免召回成本是推动T&T分析投资的驱动因素。
- 市场限制
- 各国时间表的差异会造成投资不确定性。
- 传统MES/ERP和包装生产线的高昂资本和整合成本
- 网路化溯源平台中的资料隐私与网路安全风险
- 低利润学名药生产商的投资报酬率有限。
- 技术展望
- 波特五力模型
- 新进入者的威胁
- 买方的议价能力
- 供应商的议价能力
- 替代品的威胁
- 竞争对手之间的竞争
第五章 市场规模与成长预测
- 按组件
- 硬体系统
- 印刷和标记设备
- 监控与检验系统
- 标籤和防篡改解决方案
- 其他硬体
- 软体解决方案
- 工厂级管理套件
- 线路控制器软体
- 捆包/托盘追踪软体
- 企业及云端平台
- 专业及管理服务
- 硬体系统
- 透过技术
- 条码/二维资料矩阵
- RFID和NFC
- 先进的物联网感测器和蓝牙低功耗信标
- 透过使用
- 序列化解决方案
- 瓶子序列化
- 泡壳和条状包装序列化
- 纸箱和包装箱序列化
- 资料矩阵/QR码序列化
- 聚合解决方案
- 捆绑聚合
- 病例汇总
- 调色板聚合
- 序列化解决方案
- 最终用户
- 製药公司
- 合约製造组织/CPO
- 医疗设备製造商
- 医疗保健分销商和批发商
- 其他生命科学相关利益者(非处方药、营养保健品、化妆品、合法大麻)
- 按地区
- 北美洲
- 美国
- 加拿大
- 墨西哥
- 欧洲
- 德国
- 英国
- 法国
- 义大利
- 西班牙
- 其他欧洲地区
- 亚太地区
- 中国
- 日本
- 印度
- 韩国
- 澳洲
- 亚太其他地区
- 中东和非洲
- GCC
- 南非
- 其他中东和非洲地区
- 南美洲
- 巴西
- 阿根廷
- 其他南美洲国家
- 北美洲
第六章 竞争情势
- 市场集中度
- 市占率分析
- 公司简介
- OPTEL Group
- TraceLink Inc.
- Antares Vision SpA
- SEA Vision SRL
- Syntegon Technology GmbH
- Zebra Technologies Corp.
- Mettler-Toledo International Inc.
- Korber Medipak Systems GmbH
- ACG Worldwide
- VISIOTT
- Uhlmann
- Sato Holdings Corporation
- 74Software
- Siemens
- Brother Industries, Ltd.(Domino Printing Sciences plc)
- Dover Corporation(Markem-Imaje)
- Wipotec-OCS GmbH
- Veralto Corporation(Videojet Technologies Inc.)
- Coesia SpA
第七章 市场机会与未来展望
The track & trace solutions market is valued at USD 3.51 billion in 2025 and is projected to reach USD 5.43 billion by 2030, translating into a 9.14% compound annual growth rate (CAGR).

Heightened regulatory pressure across pharmaceutical supply chains is turning serialization from a compliance chore into a strategic differentiator, prompting companies to invest in more sophisticated, data-rich platforms. As serialization and aggregation converge on cloud architectures, vendors are eyeing fresh data-monetization avenues while simultaneously confronting new cybersecurity risks. Meanwhile, the Track & Trace Solutions market share of smaller niche vendors is expanding in emerging regions because modular cloud architecture allows them to compete without a global sales footprint.
Global Track And Trace Solutions Market Trends and Insights
Convergence of Global Pharmaceutical Traceability Mandates
Momentum toward harmonized standards is compelling firms to design systems that satisfy multiple jurisdictions in a single deployment. Vendors therefore invest heavily in flexible data models that map country codes to a common core, which quietly reduces lifetime integration costs for multinational plants. One evident consequence is that manufacturers now issue global requests for proposal instead of region-specific tenders, concentrating purchasing power and accelerating vendor consolidation. This regulatory convergence simultaneously shortens rollout cycles because configuration replaces custom coding, allowing quicker payback periods and freeing budgets for advanced analytics.
Escalating Counterfeit Drug Threat Elevating Patient-Safety Imperatives
The rising sophistication of counterfeit networks forces brand owners to look beyond serialization and adopt multi-layer security that joins tamper-evident packaging, authentication algorithms, and real-time verification portals. By embedding unique identifiers inside layered packaging, companies are discouraging illicit repackaging, a fresh deterrent that also minimizes recall scope when an incident occurs. Health agencies publicly link counterfeit suppression to improved therapy adherence, which drives political endorsement of stricter serialization audits. As a direct result, solution vendors report an uptick in demand for mobile inspection apps that allow frontline staff to validate codes at every hand-off.
Divergent Country-Level Timelines Generating Investment Uncertainty
Uneven regulatory calendars compel firms to stage capital allocations carefully, often prioritizing export markets over domestic ones to protect revenue streams. Finance teams increasingly evaluate serialization projects through real-options analysis, treating each regional rollout as a sequential option rather than a deterministic plan, a mindset shift that influences vendor contracting terms. Solution providers respond by offering modular licensing that activates only when a country deadline approaches, a pricing innovation that shortens sales cycles.
Other drivers and restraints analyzed in the detailed report include:
- Surge in Direct-to-Patient and E-commerce Channels Requiring End-to-End Visibility
- Digitalization of Pharma Supply Chains and Cloud-Native SaaS Adoption
- High Capital and Integration Costs with Legacy MES, ERP, and Packaging Lines
For complete list of drivers and restraints, kindly check the Table Of Contents.
Segment Analysis
Software claimed a 52.64% track and trace solutions market size in 2024 and is projected to grow at a 8.63% CAGR through 2030. The acceleration reflects a shift toward value extraction from serialization data as firms seek operational insights and not just compliance records. Platform vendors increasingly bundle professional services, which offsets the skills gap in mid-tier manufacturers and increases switching costs. A notable pattern is that successful deployments now start with a data-governance workshop, illustrating the convergence of IT and quality functions inside pharmaceutical firms.
Hardware still anchors initial compliance, yet its track and trace solutions market share will likely be above 29.77% by 2030 as capital cycles complete. Printing, vision inspection, and tamper-evidence modules remain essential, and vendors differentiate through modularity that reduces line downtime during retrofits. Demand for RFID printers and on-printer verification grows fastest inside the hardware basket, mirroring the gradual sensorization of packaging lines. A secondary effect is that resale markets for second-generation printers gain liquidity, lowering entry barriers for late adopters in emerging economies.
Barcodes held a commanding 55.76% track and trace solutions market share in 2024, yet RFID solutions are forecast to expand at 9.90% CAGR until 2030, closing the gap steadily. Pharmaceutical warehouses, eager to cut manual scans, now pilot mixed tagging schemes in which high-value biologics carry RFID while generics retain 2D codes, demonstrating a hybrid approach that balances cost and capability. Real-time location data from RFID pallets feeds WMS algorithms that slash pick times, a benefit that quietly offsets tag costs in large distribution centers.
The resilience of barcodes stems from ubiquitous scanners and consistent regulatory acceptance, keeping the technology relevant for the foreseeable future. However, the falling price of passive UHF inlays suggests that the inflection point for wider RFID adoption is drawing nearer. As proof, several national health services request RFID for hospital logistics, creating a pull effect back up the supply chain. This developing preference nudges packaging-line OEMs to embed RFID tunnels in standard equipment offerings.
The Track and Trace Solutions Market is Segmented by Component (Hardware Systems [Printing & Marking Equipment, and More], Software Solutions, and More), Technology (Barcode / 2-D DataMatrix, and More), Application (Serialization Solutions and Aggregation Solutions), End User (Pharmaceutical Manufacturers, and More), and Geography (North America, Europe, and More). The Market Forecasts are Provided in Terms of Value (USD).
Geography Analysis
North America, with 42.24% Track & Trace Solutions market share in 2024, remains the largest region because of the Drug Supply Chain Security Act that mandates unit-level traceability by August 2025. A notable consequence is that even small generics firms accelerate projects to avoid supply interruptions, which inflates near-term demand for validation services. The region's mature electronic health-record infrastructure enables linkage between serialization data and clinical outcomes, providing a feedback loop that informs value-based contracting. This linkage also stimulates analytics investments by insurers aiming to verify adherence in specialty therapies. Large-scale wholesalers integrate serialization with robotics, reducing manual case handling and reshaping labor allocation across distribution centers.
Asia-Pacific shows the fastest trajectory at a forecast 10.29% CAGR, underpinned by aggressive anti-counterfeit campaigns in China and export incentives in India. Governments often couple local content rules with traceability grants, encouraging domestic IT firms to partner with global solution providers. Multinational manufacturers, confronted with compressed timelines, select configurable cloud platforms that can flip on country modules as rules mature. The same architecture supports language localization, a subtle yet critical success factor across a linguistically diverse region. Rapid e-commerce expansion forces regulators to tighten parcel-level verification, injecting further urgency into adoption.
Europe retains a significant position due to the mature Falsified Medicines Directive, which pushes verification down to the pharmacy counter. National medicine verification organization databases, already operational, anchor a dense scanning network that covers nearly every dispensing point. Manufacturers adapt by embedding tamper-evident seals on cartons, a design choice that raises consumer expectations globally. The region's next focus is environmental sustainability, and stakeholders explore how serialization data can support carbon-footprint reporting, demonstrating the multi-dimensional potential of track and trace infrastructure.
- OPTEL Group
- TraceLink
- Antares Vision SpA
- SEA Vision SRL
- Syntegon Technology
- Zebra Technologies
- Mettler Toledo
- Korber
- ACG Worldwide
- VISIOTT
- Uhlmann
- Sato Holdings Corporation
- 74Software
- Siemens Healthineers
- Brother Industries, Ltd. (Domino Printing Sciences plc)
- Dover Corporation (Markem-Imaje)
- Wipotec-OCS GmbH
- Veralto Corporation (Videojet Technologies Inc.)
- Coesia S.p.A.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 Introduction
- 1.1 Study Assumptions & Market Definition
- 1.2 Scope of the Study
2 Research Methodology
3 Executive Summary
4 Market Landscape
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Convergence of Global Pharmaceutical Traceability Mandates (WHO, ISO)
- 4.2.2 Escalating Counterfeit Drug Threat Elevating Patient?Safety Imperatives
- 4.2.3 Surge in Direct-to-Patient & E-commerce Channels Requiring End-to-End Visibility
- 4.2.4 Digitalization of Pharma Supply Chains and Cloud-native SaaS Adoption
- 4.2.5 Transition to Personalized & Small-Batch Therapies Necessitating Flexible Serialization
- 4.2.6 Brand Reputation & Recall Cost Avoidance Driving Investment in Track & Trace Analytics
- 4.3 Market Restraints
- 4.3.1 Divergent Country-level Timelines Generating Investment Uncertainty
- 4.3.2 High Capital & Integration Costs with Legacy MES/ERP and Packaging Lines
- 4.3.3 Data Privacy & Cyber-security Risks in Networked Traceability Platforms
- 4.3.4 Limited Per-unit ROI for Low-margin Generic Manufacturers
- 4.4 Technological Outlook
- 4.5 Porter's Five Forces
- 4.5.1 Threat of New Entrants
- 4.5.2 Bargaining Power of Buyers
- 4.5.3 Bargaining Power of Suppliers
- 4.5.4 Threat of Substitutes
- 4.5.5 Intensity of Competitive Rivalry
5 Market Size & Growth Forecasts (Value, USD)
- 5.1 By Component
- 5.1.1 Hardware Systems
- 5.1.1.1 Printing & Marking Equipment
- 5.1.1.2 Monitoring & Verification Systems
- 5.1.1.3 Labeling & Tamper-evident Solutions
- 5.1.1.4 Other Hardware
- 5.1.2 Software Solutions
- 5.1.2.1 Plant-level Management Suites
- 5.1.2.2 Line Controller Software
- 5.1.2.3 Bundle / Pallet Tracking Software
- 5.1.2.4 Enterprise & Cloud Platforms
- 5.1.3 Professional & Managed Services
- 5.1.1 Hardware Systems
- 5.2 By Technology
- 5.2.1 Barcode / 2-D DataMatrix
- 5.2.2 RFID & NFC
- 5.2.3 Advanced IoT Sensors & BLE Beacons
- 5.3 By Application
- 5.3.1 Serialization Solutions
- 5.3.1.1 Bottle Serialization
- 5.3.1.2 Blister & Strip Serialization
- 5.3.1.3 Carton & Case Serialization
- 5.3.1.4 Data-Matrix / QR Serialization
- 5.3.2 Aggregation Solutions
- 5.3.2.1 Bundle Aggregation
- 5.3.2.2 Case Aggregation
- 5.3.2.3 Pallet Aggregation
- 5.3.1 Serialization Solutions
- 5.4 By End User
- 5.4.1 Pharmaceutical Manufacturers
- 5.4.2 Contract Manufacturing & Packaging Organizations (CMOs/CPOs)
- 5.4.3 Medical Device Manufacturers
- 5.4.4 Healthcare Distributors & Wholesalers
- 5.4.5 Other Life-science Stakeholders (OTC, Nutraceuticals, Cosmetics, Legal Cannabis)
- 5.5 Geography
- 5.5.1 North America
- 5.5.1.1 United States
- 5.5.1.2 Canada
- 5.5.1.3 Mexico
- 5.5.2 Europe
- 5.5.2.1 Germany
- 5.5.2.2 United Kingdom
- 5.5.2.3 France
- 5.5.2.4 Italy
- 5.5.2.5 Spain
- 5.5.2.6 Rest of Europe
- 5.5.3 Asia-Pacific
- 5.5.3.1 China
- 5.5.3.2 Japan
- 5.5.3.3 India
- 5.5.3.4 South Korea
- 5.5.3.5 Australia
- 5.5.3.6 Rest of Asia-Pacific
- 5.5.4 Middle East and Africa
- 5.5.4.1 GCC
- 5.5.4.2 South Africa
- 5.5.4.3 Rest of Middle East and Africa
- 5.5.5 South America
- 5.5.5.1 Brazil
- 5.5.5.2 Argentina
- 5.5.5.3 Rest of South America
- 5.5.1 North America
6 Competitive Landscape
- 6.1 Market Concentration
- 6.2 Market Share Analysis
- 6.3 Company profiles (includes Global level Overview, Market level overview, Core Segments, Financials as available, Strategic Information, Market Rank/Share for key companies, Products & Services, and Recent Developments)
- 6.3.1 OPTEL Group
- 6.3.2 TraceLink Inc.
- 6.3.3 Antares Vision SpA
- 6.3.4 SEA Vision SRL
- 6.3.5 Syntegon Technology GmbH
- 6.3.6 Zebra Technologies Corp.
- 6.3.7 Mettler-Toledo International Inc.
- 6.3.8 Korber Medipak Systems GmbH
- 6.3.9 ACG Worldwide
- 6.3.10 VISIOTT
- 6.3.11 Uhlmann
- 6.3.12 Sato Holdings Corporation
- 6.3.13 74Software
- 6.3.14 Siemens
- 6.3.15 Brother Industries, Ltd. (Domino Printing Sciences plc)
- 6.3.16 Dover Corporation (Markem-Imaje)
- 6.3.17 Wipotec-OCS GmbH
- 6.3.18 Veralto Corporation (Videojet Technologies Inc.)
- 6.3.19 Coesia S.p.A.
7 Market Opportunities & Future Outlook
- 7.1 White-space & Unmet-need Assessment













