 |
市场调查报告书
商品编码
1723660
RSV疫苗市场:疫苗类别,各给药途径,对象患者层,各流通管道,主要各地区,各主要企业:2040年为止的产业趋势与全球预测RSV Vaccine Market by Type of Vaccine, Route of Administration, Target Patient Population, Distribution Channel, Key Geographical Regions, and Leading Players: Industry Trends and Global Forecasts, till 2040 |
||||||
RSV 疫苗市场
今年全球 RSV 疫苗市场规模为 11.49 亿美元。预计到2040年,市场规模将达到7.31亿美元。
呼吸道合胞病毒(RSV)疫苗市场机会分布在以下领域:
疫苗类型
- 次单元/类病毒颗粒(VLP)疫苗
- 活病毒疫苗
- mRNA疫苗
给药途径
- 肌肉内
- 其他
对象患者层
- 老年人
- 婴幼儿/儿童
- 孕妇
流通管道
- 药局·药店
- 政府/机关供应商
- 其他
主要地区
- 北美
- 欧洲
呼吸道合胞病毒 (RSV) 疫苗市场:成长与趋势
呼吸道合胞病毒 (RSV) 是一种常见病毒,会在婴儿、老年人和免疫功能低下人群中传播下呼吸道感染。该病毒被认为是一种高度传染性的呼吸道病原体,在世界各地导致严重疾病和死亡。根据世界卫生组织统计,全球每年约有 3,300 万例 5 岁以下儿童新发 RSV 感染病例。这是由于缺乏有效的 RSV 感染治疗方法。
目前,包括非处方药、滴鼻剂和加湿器在内的多种预防方法被用于治疗 RSV 感染。然而,这些只是症状治疗,因此疫苗因其为患者提供安全有效的针对性治疗而成为一种有前景的选择。这些疫苗有望最大程度地降低呼吸道合胞病毒感染以及支气管炎和肺炎等呼吸道疾病相关的住院率、併发症和死亡率。

由于 mRNA 和奈米颗粒技术等技术进步,这些疫苗能够提供长期免疫力并有效治疗呼吸道合胞病毒感染。此外,随着研发投入和 RSV 治疗临床试验数量的增加,预计 RSV 疫苗的需求将会增加,这可能会推动市场在未来几年实现健康成长。
呼吸道合胞病毒 (RSV) 疫苗市场:关键洞察
本报告分析了呼吸道合胞病毒 (RSV) 疫苗市场的现状,并探讨了该行业的潜在成长机会。主要发现包括:
- 目前,有超过 70 种呼吸道合胞病毒 (RSV) 疫苗正在使用各种技术进行研发,其中超过 60% 的疫苗正处于不同的临床试验阶段。
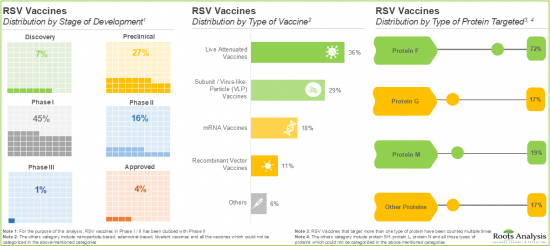
- 呼吸道合胞病毒 (RSV) 疫苗需求的不断增长促使疫苗研发管线显着增加,目前约有 65 种疫苗处于临床前和临床研发阶段。
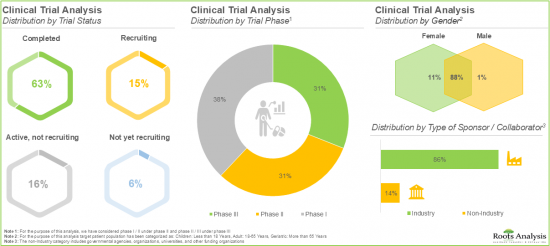
- 超过 85% 的呼吸道合胞病毒 (RSV) 疫苗临床试验由业内公司赞助。
- 自 2020 年以来,RSV 疫苗领域已提交/授予超过 330 项专利,其中大部分专利申请于北美(超过 50%)。
- 日益严重的呼吸道合胞病毒感染疫情和技术进步的步伐加快,推动着 RSV 疫苗市场的发展,预计该市场在短期内将实现稳步增长。
- 预计 RSV 疫苗市场的整体机会将涵盖各个关键地区、疫苗类型、给药途径、目标患者群体和分销管道。
RSV 疫苗市场:主要细分市场
依疫苗类型,全球 RSV 疫苗市场分为次单位/病毒样颗粒 (VLP) 疫苗、减毒活疫苗和 mRNA 疫苗。目前,亚单位/病毒样颗粒 (VLP) 疫苗占据 RSV 疫苗市场的大部分占有率。这是因为这些疫苗在形态上与母体病毒相似,产生强烈的免疫原性反应。
依给药途径,全球 RSV 疫苗市场分为肌肉注射和其他途径。目前,透过肌肉注射途径接种的疫苗可能占据整个市场的主导地位。这归功于其高生物利用度,确保高效吸收并减少副作用,使其成为患者更安全、更有效的选择。
全球呼吸道合胞病毒疫苗市场按目标患者族群细分为老年人、婴幼儿及孕妇。目前,呼吸道合胞病毒疫苗市场将以老年人群为主。这是因为老年人极易感染呼吸道疾病,尤其是在呼吸道合胞病毒高发生期。
全球呼吸道合胞病毒疫苗市场按分销管道细分为药局、政府和机构供应商以及其他管道。值得注意的是,在预测期内,药局通路很可能占据呼吸道合胞病毒疫苗市场的主导地位。这是因为该疫苗接种方便、普及,且药师对疫苗接种的认可度不断提高,从而推动了疫苗接种率的提高。
依主要地区划分,市场分为北美和欧洲。目前,北美占据最大的市场占有率,这归因于该地区呼吸道感染病例的不断增加、完善的监管框架以及先进的医疗基础设施。
报告解答的关键问题
- 目前有多少家公司进入该市场?
- 该市场的主要公司
- 可能影响该市场发展的因素
- 当前与未来的市场规模
- 该市场的复合年增长率
- 当前和未来的市场机会如何在主要细分市场中分配?
- 市场专利申请趋势
为何购买此报告?
- 本报告提供全面的市场分析,并针对整体市场和特定细分市场提供详细的收入预测。这些资讯对于现有的市场领导者和新进入者都极具价值。
- 利害关係人可以利用本报告深入了解市场竞争动态。分析竞争格局有助于企业做出明智的决策,优化市场定位,并制定有效的市场进入策略。
- 本报告为利害关係人提供了全面的市场概览,包括关键推动因素、阻碍因素和挑战。这些资讯使利害关係人能够掌握市场趋势,并做出基于数据的决策,从而掌握成长前景。
更多优势
- 报告中所有分析模组均提供免费 Excel 资料包
- 10% 免费内容客製化
- 由我们的研究团队提供详细的报告讲解
- 如果报告发布时间超过 6-12 个月,则可免费更新报告
按本报告提供全球RSV疫苗市场相关调查,提供市场概要,以及疫苗类别,各给药途径,对象患者层,各流通管道,主要各地区,各主要企业趋势,及加入此市场的主要企业简介等资讯。
目录
章节I:报告概要
第1章 背景
第2章 调查手法
第3章 市场动态
第4章 宏观经济指标
章节II:定性性的洞察
第5章 摘要整理
第6章 简介
- 呼吸道融合病毒(RSV)疫苗的简介
- RSV的结构与机制
- 呼吸道融合病毒(RSV)疫苗相关的主要历史的事件
- 呼吸道融合病毒(RSV)疫苗类型
- 呼吸道融合病毒(RSV)疫苗接种的优点
- 呼吸道融合病毒(RSV)疫苗伴随开发的课题
- 未来展望
章节III:市场概要
第7章 市场形势
- 呼吸道融合病毒(RSV)疫苗:市场形势
第8章 产品竞争力分析
- 前提主要的参数
- 调查手法
- 呼吸道融合病毒(RSV)疫苗:产品竞争力分析
章节IV:企业简介
第9章 企业简介北美的呼吸道融合病毒(RSV)疫苗开发企业
- Icosavax(A Company of AstraZeneca)
- Moderna
- Pfizer
第10章 企业简介:欧洲的RSV疫苗开发企业
- GlaxoSmithKline
- Sanofi
第11章 企业简介:亚太地区的RSV疫苗开发企业
- Beijing Advaccine Biotechnology
第5章 市场趋势
第12章 临床试验的分析
- 范围调查手法
- 呼吸道融合病毒(RSV)疫苗:临床试验分析
第13章 专利分析
第14章 FDA核准策略
章节VI:市场机会分析
第15章 市场影响分析:促进因素,阻碍因素,机会,课题
- 市场促进因素
- 市场阻碍因素
- 市场机会
- 市场课题
第16章 全球RSV疫苗市场
第17章 呼吸道融合病毒(RSV)疫苗市场(疫苗类别)
第18章 呼吸道融合病毒(RSV)疫苗市场(各给药途径)
第19章 呼吸道融合病毒(RSV)疫苗市场(对象患者层)
第20章 呼吸道融合病毒(RSV)疫苗市场(各流通管道)
第21章 呼吸道融合病毒(RSV)疫苗市场(主要各地区)
第22章 市场集中分析(主要加入企业)
章节VII:地理地区的市场机会分析
第23章 市场机会分析:北美
第24章 市场机会分析:欧洲
章节VIII:其他的垄断的洞察
第25章 结论
第26章 执行洞察
章节IX:附录
第27章 表格形式资料
第28章 企业·团体一览
RSV VACCINE MARKET
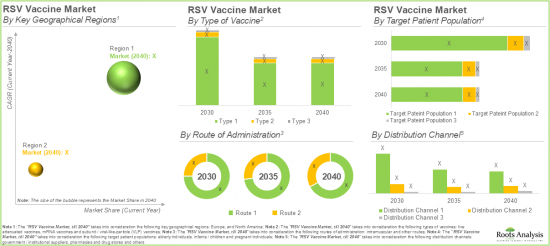
As per Roots Analysis, the global RSV vaccine market size is worth USD 1,149 million in the current year and is expected to be worth USD 731 million by 2040.
The opportunity for RSV vaccine market has been distributed across the following segments:
Type of Vaccine
- Subunit / Viral-like-Particle (VLP) Vaccines
- Live Attenuated Vaccines
- mRNA Vaccines
Route of Administration
- Intramuscular
- Other Routes
Target Patient Population
- Elderly Individuals
- Infants / Children
- Pregnant Individuals
Distribution Channel
- Pharmacies and Drug Stores
- Government / Institutional Suppliers
- Others
Key Geographical Regions
- North America
- Europe
RSV VACCINE MARKET: GROWTH AND TRENDS
Respiratory Syncytial Virus (RSV) is a common virus spreading lower respiratory tract infections in infants, older adults, and immunocompromised individuals. This virus is recognized as a highly contagious respiratory pathogen, which contributes to severe illness and mortality across the globe. According to the WHO, around 33 million new cases of RSV infections are emerging annually in children under the age of five across the globe. This can be attributed to the lack of effective treatment options for RSV infections.
Currently, several preventive measures, such as over-the-counter medications, nasal sprays, and humidifiers are being used for the treatment of RSV infections. However, they only provide symptomatic relief, making vaccines a promising alternative that offers safe, effective and targeted treatment to patients. These vaccines have the potential to minimize hospitalization rates, complications, and mortality rates related to RSV infections as well as other respiratory illnesses, such as bronchitis and pneumonia.

Driven by technological advancements, including the use of mRNA and nanoparticle technology for developing RSV vaccines, these vaccines are providing long lasting immunity and effective treatment against RSV infections. Moreover, owing to the increased research and development efforts and rising number of clinical trials for RSV treatment, the demand for RSV vaccines is anticipated to rise, positioning the market for healthy growth in the forthcoming years.
RSV VACCINE MARKET: KEY INSIGHTS
The report delves into the current state of the RSV vaccine market and identifies potential growth opportunities within the industry. Some key findings from the report include:
- Presently, more than 70 RSV vaccines are being developed using various technologies; notably, over 60% of these vaccines are under investigation in different clinical trial phases.

- The rising demand for RSV vaccines has led to a notable increase in the vaccine pipeline, with around 65 vaccines currently under preclinical and clinical stages of development.
- A sizeable increase in the number of registered clinical trials has been observed in recent years; over 85% of the trials evaluating RSV vaccines have been sponsored by industry players.

- Since 2020, more than 330 patents have been filed / granted in the RSV vaccines domain; further, the majority of the patents were filed in North America (>50%).
- The growing prevalence of respiratory syncytial virus infections, coupled with the increasing pace of technological advancements is driving the RSV vaccines market and positioning it for steady growth in the foreseeable future.
- The overall opportunity within the RSV vaccine market is anticipated to be well distributed across various key geographical regions, types of vaccines, routes of administration, target patient populations and distribution channels.

RSV VACCINE MARKET: KEY SEGMENTS
Subunit / Viral-Like-Particle (VLP) Vaccines are Likely to Dominate the RSV Vaccine Market During the Forecast Period
Based on the types of vaccines, the global RSV vaccine market is segmented into subunit / viral-like-particle (VLP) vaccines, live attenuated vaccines and mRNA vaccines. Currently, the majority share of the RSV vaccine market is captured by subunit / Viral-Like-Particle (VLP) vaccines, owing to the strong immunogenic response produced by these vaccines since they are morphologically similar to their parent virus.
Vaccines Administered through Intramuscular Route are Likely to Hold the Largest Share of the RSV Vaccine Market during the Forecast Period
Based on the route of administration, the global RSV vaccine market is distributed across intramuscular and other routes. Currently, the vaccines administered through the intramuscular route are likely to dominate the overall market. This can be attributed to their high bioavailability, which ensures efficient absorption, along with reduced side effects, making them a safer and more effective option for patients.
By Target Patient Population, RSV Vaccines Developed for Elderly Individuals are Likely to Dominate the Market during the Forecast Period
Based on the target patient population, the global RSV vaccine market is segmented into elderly individuals, infants / children and pregnant individuals. The current RSV vaccine market is likely to be dominated by elderly individuals' segment. This can be attributed to the fact that elderly individuals are highly susceptible to respiratory diseases, specifically during RSV season.
By Type of Material, Stainless Steel Segment is Likely to Dominate the Market During the Forecast Period
Based on the distribution channel, the global RSV vaccine market is segmented into pharmacies and drug stores, government / institutional suppliers and others. Notably, the pharmacies and drug stores segment are likely to dominate the RSV vaccine market during the forecast period. This can be attributed to their convenience and widespread accessibility, along with the rising authorization of pharmacists to administer vaccines which has contributed to increased vaccination rates.
North America Accounts for the Largest Share of the Market
Based on key geographical regions, the market is segmented into North America and Europe. In the current scenario, North America is likely to capture the largest market share which can be attributed to the growing incidences of respiratory infections, robust regulatory frameworks, and advanced healthcare infrastructure in this region.
Example Players in the RSV Vaccine Market
- AIM Vaccine
- Beijing Advaccine Biotechnology
- CureVac
- Daiichi Sankyo
- GlaxoSmithKline
- Icosavax (A company of AstraZeneca)
- Johnson & Johnson
- KM Biologics
- MedImmune (A part of AstraZeneca)
- Moderna
- Pfizer
- Sanofi
- SK Bioscience
RSV VACCINE MARKET: RESEARCH COVERAGE
The report on the RSV vaccine market features insights on various sections, including:
- Market Sizing and Opportunity Analysis: An in-depth analysis of the RSV vaccine market, focusing on key market segments, including [A] type of vaccine, [B] route of administration, [C] target patient population, [D] distribution channel, and [E] key geographical regions and [F] leading players.
- Market Impact Analysis: A thorough analysis of various factors, such as drivers, restraints, opportunities, and existing challenges that are likely to impact market growth.
- RSV Vaccines Market Landscape: A comprehensive evaluation of various RSV vaccines, based on several relevant parameters, such as [A] stage of development, [B] route of administration, [C] type of vaccine, [D] type of protein targeted, [E] type of immunization, and [F] target patient population. This section also includes an evaluation of the companies engaged in developing RSV vaccines, based on several relevant parameters, such as [A] year of establishment, [B] company size, [C] location of headquarters.
- Product Competitiveness Analysis: An insightful competitiveness analysis of various RSV vaccines based on various relevant parameters, such as [A] developer strength, [B] product competitiveness, and [C] portfolio diversity.
- Company Profiles: Elaborate profiles of prominent RSV vaccine developers across various geographies, including North America, Europe, Asia-Pacific, providing details on [A] company overview, [B] financial information (if available), [C] product portfolio, [D] recent developments and [E] an informed future outlook.
- Clinical Trial Analysis: A detailed assessment of clinical trials that have been published for various types of RSV vaccines, based on various relevant parameters, such as [A] trial registration year, [B] trial status, [C] trial phase, [D] study design, [E] type of sponsor / collaborator, [F] gender, [G] target patient population, [H] leading players and [I] geography.
- Patent Analysis: An in-depth analysis of patents filed / granted till date in the RSV vaccine domain, based on various relevant parameters, such as [A] type of patent, [B] publication year, [C] patent application year, [D] CPC symbols, [E] patent jurisdiction, [F] type of applicant, [G] leading industry players, [H] leading industry players, [I] patent benchmarking, [J] patent age, and [K] patent valuation analysis.
- FDA Approval Strategies: A detailed overview of various competitive strategies that can be incorporated by the RSV vaccine developers in order to expedite the FDA approval process of their proprietary vaccines. The section also provides information on several stakeholders undertaking various initiatives, including awards / grants, partnerships, and expanding intellectual properties.
KEY QUESTIONS ANSWERED IN THIS REPORT
- How many companies are currently engaged in this market?
- Which are the leading companies in this market?
- What factors are likely to influence the evolution of this market?
- What is the current and future market size?
- What is the CAGR of this market?
- How is the current and future market opportunity likely to be distributed across key market segments?
- What is the patent filing activity trend in the market?
REASONS TO BUY THIS REPORT
- The report provides a comprehensive market analysis, offering detailed revenue projections of the overall market and its specific sub-segments. This information is valuable to both established market leaders and emerging entrants.
- Stakeholders can leverage the report to gain a deeper understanding of the competitive dynamics within the market. By analyzing the competitive landscape, businesses can make informed decisions to optimize their market positioning and develop effective go-to-market strategies.
- The report offers stakeholders a comprehensive overview of the market, including key drivers, barriers, opportunities, and challenges. This information empowers stakeholders to stay abreast of market trends and make data-driven decisions to capitalize on growth prospects.
ADDITIONAL BENEFITS
- Complimentary Excel Data Packs for all Analytical Modules in the Report
- 10% Free Content Customization
- Detailed Report Walkthrough Session with Research Team
- Free Updated report if the report is 6-12 months old or older
TABLE OF CONTENTS
SECTION I: REPORT OVERVIEW
1. BACKGROUND
- 1.1. Context
- 1.2. Project Objectives
2. RESEARCH METHODOLOGY
- 2.1. Chapter Overview
- 2.2. Research Assumptions
- 2.2.1. Market Landscape and Market Trends
- 2.2.2. Market Forecast and Opportunity Analysis
- 2.2.3. Comparative Analysis
- 2.3. Database Building
- 2.3.1. Data Collection
- 2.3.2. Data Validation
- 2.3.3. Data Analysis
- 2.4. Project Methodology
- 2.4.1. Secondary Research
- 2.4.1.1. Annual Reports
- 2.4.1.2. Academic Research Papers
- 2.4.1.3. Company Websites
- 2.4.1.4. Investor Presentations
- 2.4.1.5. Regulatory Filings
- 2.4.1.6. White Papers
- 2.4.1.7. Industry Publications
- 2.4.1.8. Conferences and Seminars
- 2.4.1.9. Government Portals
- 2.4.1.10. Media and Press Releases
- 2.4.1.11. Newsletters
- 2.4.1.12. Industry Databases
- 2.4.1.13. Roots Proprietary Databases
- 2.4.1.14. Paid Databases and Sources
- 2.4.1.15. Social Media Portals
- 2.4.1.16. Other Secondary Sources
- 2.4.2. Primary Research
- 2.4.2.1. Types of Primary Research
- 2.4.2.1.1. Qualitative Research
- 2.4.2.1.2. Quantitative Research
- 2.4.2.1.3. Hybrid Approach
- 2.4.2.2. Advantages of Primary Research
- 2.4.2.3. Techniques for Primary Research
- 2.4.2.3.1. Interviews
- 2.4.2.3.2. Surveys
- 2.4.2.3.3. Focus Groups
- 2.4.2.3.4. Observational Research
- 2.4.2.3.5. Social Media Interactions
- 2.4.2.4. Key Opinion Leaders Considered in Primary Research
- 2.4.2.4.1. Company Executives (CXOs)
- 2.4.2.4.2. Board of Directors
- 2.4.2.4.3. Company Presidents and Vice Presidents
- 2.4.2.4.4. Research and Development Heads
- 2.4.2.4.5. Technical Experts
- 2.4.2.4.6. Subject Matter Experts
- 2.4.2.4.7. Scientists
- 2.4.2.4.8. Doctors and Other Healthcare Providers
- 2.4.2.5. Ethics and Integrity
- 2.4.2.5.1. Research Ethics
- 2.4.2.5.2. Data Integrity
- 2.4.2.1. Types of Primary Research
- 2.4.3. Analytical Tools and Databases
- 2.4.1. Secondary Research
- 2.5. Robust Quality Control
3. MARKET DYNAMICS
- 3.1. Chapter Overview
- 3.2. Forecast Methodology
- 3.2.1. Top-down Approach
- 3.2.2. Bottom-up Approach
- 3.2.3. Hybrid Approach
- 3.3. Market Assessment Framework
- 3.3.1. Total Addressable Market (TAM)
- 3.3.2. Serviceable Addressable Market (SAM)
- 3.3.3. Serviceable Obtainable Market (SOM)
- 3.3.4. Currently Acquired Market (CAM)
- 3.4. Forecasting Tools and Techniques
- 3.4.1. Qualitative Forecasting
- 3.4.2. Correlation
- 3.4.3. Regression
- 3.4.4. Extrapolation
- 3.4.5. Convergence
- 3.4.6. Sensitivity Analysis
- 3.4.7. Scenario Planning
- 3.4.8. Data Visualization
- 3.4.9. Time Series Analysis
- 3.4.10. Forecast Error Analysis
- 3.5. Key Considerations
- 3.5.1. Demographics
- 3.5.2. Government Regulations
- 3.5.3. Reimbursement Scenarios
- 3.5.4. Market Access
- 3.5.5. Supply Chain
- 3.5.6. Industry Consolidation
- 3.5.7. Pandemic / Unforeseen Disruptions Impact
- 3.6. Limitations
4. MACRO-ECONOMIC INDICATORS
- 4.1. Chapter Overview
- 4.2. Market Dynamics
- 4.2.1. Time Period
- 4.2.1.1. Historical Trends
- 4.2.1.2. Current and Forecasted Estimates
- 4.2.2. Currency Coverage
- 4.2.2.1. Major Currencies Affecting the Market
- 4.2.2.2. Factors Affecting Currency Fluctuations in the Industry
- 4.2.2.3. Impact of Currency Fluctuations on the Industry
- 4.2.3. Foreign Currency Exchange Rate
- 4.2.3.1. Impact of Foreign Exchange Rate Volatility on the Market
- 4.2.3.2. Strategies for Mitigating Foreign Exchange Risk
- 4.2.4. Recession
- 4.2.4.1. Assessment of Current Economic Conditions and Potential Impact on the Market
- 4.2.4.2. Historical Analysis of Past Recessions and Lessons Learnt
- 4.2.5. Inflation
- 4.2.5.1. Measurement and Analysis of Inflationary Pressures in the Economy
- 4.2.5.2. Potential Impact of Inflation on the Market Evolution
- 4.2.6. Interest Rates
- 4.2.6.1. Interest Rates and Their Impact on the Market
- 4.2.6.2. Strategies for Managing Interest Rate Risk
- 4.2.7. Commodity Flow Analysis
- 4.2.7.1. Type of Commodity
- 4.2.7.2. Origins and Destinations
- 4.2.7.3. Values and Weights
- 4.2.7.4. Modes of Transportation
- 4.2.8. Global Trade Dynamics
- 4.2.8.1. Import Scenario
- 4.2.8.2. Export Scenario
- 4.2.8.3. Trade Policies
- 4.2.8.4. Strategies for Mitigating the Risks Associated with Trade Barriers
- 4.2.8.5. Impact of Trade Barriers on the Market
- 4.2.9. War Impact Analysis
- 4.2.9.1. Russian-Ukraine War
- 4.2.9.2. Israel-Hamas War
- 4.2.10. COVID Impact / Related Factors
- 4.2.10.1. Global Economic Impact
- 4.2.10.2. Industry-specific Impact
- 4.2.10.3. Government Response and Stimulus Measures
- 4.2.10.4. Future Outlook and Adaptation Strategies
- 4.2.11. Other Indicators
- 4.2.11.1. Fiscal Policy
- 4.2.11.2. Consumer Spending
- 4.2.11.3. Gross Domestic Product
- 4.2.11.4. Employment
- 4.2.11.5. Taxes
- 4.2.11.6. Stock Market Performance
- 4.2.11.7. Cross Border Dynamics
- 4.2.1. Time Period
- 4.3. Conclusion
SECTION II: QUALITATIVE INSIGHTS
5. EXECUTIVE SUMMARY
6. INTRODUCTION
- 6.1. Introduction to RSV Vaccines
- 6.2. Structure and Mechanism of RSV
- 6.3. Key Historical Events Related to RSV Vaccines
- 6.4 Types of RSV Vaccines
- 6.4.1. Nucleic Acid Based Vaccines
- 6.4.2. Protein Based Vaccines
- 6.4.3. Recombinant Vector Based Vaccines
- 6.4.4. Live Attenuated Vaccines
- 6.5. Benefits of RSV Vaccination
- 6.6. Challenges Associated with Development of RSV Vaccines
- 6.7. Future Perspectives
SECTION III: MARKET OVERVIEW
7. MARKET LANDSCAPE
- 7.1. RSV Vaccines: Overall Market Landscape
- 7.1.1. Analysis by Stage of Development
- 7.1.2. Analysis by Route of Administration
- 7.1.3. Analysis by Type of Vaccine
- 7.1.4. Analysis by Type of Protein Targeted
- 7.1.5. Analysis by Type of Immunization
- 7.1.6. Analysis by Target Patient Population
- 7.1.7. Analysis by Year of Establishment
- 7.1.8. Analysis by Company Size
- 7.1.9. Analysis by Location of Headquarters
8. PRODUCT COMPETITIVENESS ANALYSIS
- 8.1. Assumptions and Key Parameters
- 8.2. Methodology
- 8.3. RSV Vaccines: Product Competitiveness Analysis
- 8.3.1. RSV Vaccines Developed by Players Based in North America
- 8.3.2. RSV Vaccines Developed by Players Based in Europe
- 8.3.3. RSV Vaccines Developed by Players Based in Asia-Pacific and Rest of the World
SECTION IV: COMPANY PROFILES
9. COMPANY PROFILES: RSV VACCINE DEVELOPERS IN NORTH AMERICA
- 9.1. Icosavax (A Company of AstraZeneca)
- 9.1.1. Company Details
- 9.1.2. Product Portfolio
- 9.1.3. Recent Developments and Future Outlook
- 9.2. Moderna
- 9.2.1. Company Details
- 9.2.2. Product Portfolio
- 9.2.3. Financial Details, FY 2021 onwards (USD Million)
- 9.2.4. Recent Developments and Future Outlook
- 9.3. Pfizer
- 9.3.1. Company Details
- 9.3.2. Product Portfolio
- 9.3.3. Financial Details, FY 2021 onwards (USD Million)
- 9.3.4. Recent Developments and Future Outlook
10. COMPANY PROFILES: RSV VACCINE DEVELOPERS IN EUROPE
- 10.1. GlaxoSmithKline
- 10.1.1. Company Details
- 10.1.2. Product Portfolio
- 10.1.3. Financial Details, FY 2021 onwards (EUR Million)
- 10.1.4. Recent Developments and Future Outlook
- 10.2. Sanofi
- 10.2.1. Company Details
- 10.2.2. Product Portfolio
- 10.2.3. Financial Details, FY 2021 onwards (EUR Million)
- 10.2.4. Recent Developments and Future Outlook
11. COMPANY PROFILES: RSV VACCINE DEVELOPERS IN ASIA-PACIFIC
- 11.1. Beijing Advaccine Biotechnology
- 11.1.1. Company Details
- 11.1.2. Product Portfolio
SECTION V: MARKET TRENDS
12. CLINICAL TRIALS ANALYSIS
- 12.1. Scope and Methodology
- 12.2. RSV Vaccines: Clinical Trial Analysis
- 12.2.1. Analysis by Trial Registration Year, Since 2015
- 12.2.2. Analysis by Trial Status
- 12.2.3. Analysis of Enrolled Patient Population by Trial Registration Year, Since 2015
- 12.2.4. Analysis by Trial Registration Year and Trial Status, Since 2015
- 12.2.5. Analysis by Trial Phase
- 12.2.6. Analysis of Enrolled Patient Population by Trial Phase
- 12.2.7. Analysis by Study Design
- 12.2.8. Analysis by Type of Sponsor / Collaborator
- 12.2.9. Analysis by Gender
- 12.2.10. Analysis by Target Patient Population
- 12.2.11. Leading Players: Analysis by Number of Registered Trials
- 12.2.12. Analysis by Geography
- 12.2.13. Analysis by Trial Status and Geography
- 12.2.14. Analysis of Enrolled Patient Population by Trial Status and Geography
13. PATENT ANALYSIS
- 13.1. Scope and Methodology
- 13.2. RSV Vaccines: Patent Analysis
- 13.2.1. Analysis by Patent Publication Year, Since 2020
- 13.2.2. Analysis by Type of Patent
- 13.2.3. Analysis by Type of Patent and Publication Year, Since 2020
- 13.2.4. Analysis by Patent Application Year, Since 2008
- 13.2.5. Analysis by Patent Age
- 13.2.6. Analysis by Type of Applicant
- 13.2.7. Analysis by Patent Jurisdiction
- 13.2.8. Analysis by CPC Symbols
- 13.2.9. Leading Industry Players: Analysis by Number of Patents
- 13.2.10. Leading Non-Industry Players: Analysis by Number of Patents
- 13.2.11. Leading Inventors: Analysis by Number of Patents
- 13.3. Patent Benchmarking Analysis
- 13.3.1. Analysis of Patent Characteristics (CPC Codes) by Leading Industry Players
- 13.3.2. Analysis of Leading Industry Players by Patent Characteristics (CPC Codes)
- 13.4. Patent Valuation
- 13.5. Leading Patents by Number of Citations
14. FDA APPROVAL STRATEGIES
- 14.1. Chapter Overview
- 14.2. Methodology
- 14.3. Key Parameters
- 14.4. General Reasons for Failure of Trials Focused on RSV vaccines
- 14.5. Benchmarking Analysis: Distribution of Key Strategies by Vaccines
SECTION VI: MARKET OPPORTUNITY ANALYSIS
15. MARKET IMPACT ANALYSIS: DRIVERS, RESTRAINTS, OPPORTUNITIES AND CHALLENGES
- 15.1. Market Drivers
- 15.2. Market Restraints
- 15.3. Market Opportunities
- 15.4. Market Challenges
16. GLOBAL RSV VACCINE MARKET
- 16.1. Key Assumptions and Methodology
- 16.2. Global RSV Vaccine Market, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040) (USD Million)
- 16.2.1. Multivariate Scenario Analysis
- 16.2.1.1. Conservative Scenario
- 16.2.1.2. Optimistic Scenario
- 16.2.1. Multivariate Scenario Analysis
- 16.3. Key Market Segmentations
17. RSV VACCINE MARKET, BY TYPE OF VACCINE
- 17.1. Key Assumptions and Methodology
- 17.2. RSV Vaccine Market: Distribution by Type of Vaccine
- 17.2.1. RSV Vaccine Market for Subunit / Viral-like-Particle (VLP) Vaccines, Historical Trends (Since2023) and Forecasted Estimates (Till 2040) (USD Million)
- 17.2.2. RSV Vaccine Market for Live Attenuated Vaccines, Historical Trends (Since2023) and Forecasted Estimates (Till 2040) (USD Million)
- 17.2.3. RSV Vaccine Market for mRNA Vaccines, Historical Trends (Since2023) and Forecasted Estimates (Till 2040) (USD Million)
- 17.3. Data Triangulation and Validation
18. RSV VACCINE MARKET, BY ROUTE OF ADMINISTRATION
- 18.1. Key Assumptions and Methodology
- 18.2. RSV Vaccine Market: Distribution by Route of Administration
- 18.2.1. RSV Vaccine Market for Intramuscular, Historical Trends (Since2023) and Forecasted Estimates (Till 2040) (USD Million)
- 18.2.2. RSV Vaccine Market for Other Routes, Historical Trends (Since2023) and Forecasted Estimates Till 2040) (USD Million)
- 18.3. Data Triangulation and Validation
19. RSV VACCINE MARKET, BY TARGET PATIENT POPULATION
- 19.1. Key Assumptions and Methodology
- 19.2. RSV Vaccine Market: Distribution by Target Patient Population
- 19.2.1. RSV Vaccine Market for Elderly Individuals, Historical Trends (Since2023) and Forecasted Estimates (Till 2040) (USD Million)
- 19.2.2. RSV Vaccine Market for Infants / Children, Historical Trends (Since2023) and Forecasted Estimates (Till 2040) (USD Million)
- 19.2.3. RSV Vaccine Market for Pregnant Individuals, Historical Trends (Since2023) and Forecasted Estimates (Till 2040) (USD Million)
- 19.3. Data Triangulation and Validation
20. RSV VACCINE MARKET, BY DISTRIBUTION CHANNEL
- 20.1. Key Assumptions and Methodology
- 20.2. RSV Vaccine Market: Distribution by Distribution Channel
- 20.2.1. RSV Vaccine Market for Pharmacies and Drug Stores, Historical Trends (Since2023) and Forecasted Estimates (Till 2040) (USD Million)
- 20.2.2. RSV Vaccine Market for Government / Institutional Suppliers, Historical Trends (Since2023) and Forecasted Estimates (Till 2040) (USD Million)
- 20.2.3. RSV Vaccine Market for Others, Historical Trends (Since2023) and Forecasted Estimates (Till 2040) (USD Million)
- 20.3. Data Triangulation and Validation
21. RSV VACCINE MARKET, BY KEY GEOGRAPHICAL REGIONS
- 21.1. Key Assumptions and Methodology
- 21.2. RSV Vaccine Market: Distribution by Key Geographical Regions
- 21.2.1. RSV Vaccine Market in North America, Historical Trends (Since2023) and Forecasted Estimates (Till 2040) (USD Million)
- 21.2.2. RSV Vaccine Market in Europe, Historical Trends (Since2023) and Forecasted Estimates (Till 2040) (USD Million)
- 21.3. Market Movement Analysis
- 21.4. Penetration-Growth (P-G) Matrix
- 21.5. Data Triangulation and Validation
22. MARKET CONCENTRATION ANALYSIS: DISTRIBUTION BY LEADING PLAYERS
- 22.1. Key Assumptions and Methodology
- 22.2. RSV Vaccine Market: Leading RSV Vaccines Developers
- 22.3. Data Triangulation and Validation
SECTION VII: MARKET OPPORTUNITY ANALYSIS WITHIN GEOGRAPHICAL REGIONS**
23. MARKET OPPORTUNITY ANALYSIS: NORTH AMERICA
- 23.1. RSV Vaccine Market in North America: Distribution by Type of Vaccine
- 23.1.1. RSV Vaccine Market in North America for Subunit / Viral-like-Particle (VLP) Vaccines, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040)
- 23.1.2. RSV Vaccine Market in North America for Live Attenuated Vaccines, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040)
- 23.1.3. RSV Vaccine Market in North America for mRNA Vaccines, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040)
- 23.2. RSV Vaccine Market in North America: Distribution by Route of Administration
- 23.2.1. RSV Vaccine Market in North America for Intramuscular, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040)
- 23.2.2. RSV Vaccine Market in North America for Other Routes, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040)
- 23.3. RSV Vaccine Market in North America: Distribution by Target Patient Population
- 23.3.1. RSV Vaccine Market in North America for Elderly Individuals, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040)
- 23.3.2. RSV Vaccine Market in North America for Infants / Children, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040)
- 23.3.3. RSV Vaccine Market in North America for Pregnant Individuals, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040)
- 23.4. RSV Vaccine Market in North America: Distribution by Distribution Channel
- 23.4.1. RSV Vaccine Market in North America for Pharmacies and Drug Stores, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040)
- 23.4.2. RSV Vaccine Market in North America for Government / Institutional Suppliers, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040)
- 23.4.3. RSV Vaccine Market in North America for Others, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040)
24. MARKET OPPORTUNITY ANALYSIS: EUROPE
- 24.1. RSV Vaccine Market in Europe: Distribution by Type of Vaccine
- 24.1.1. RSV Vaccine Market in Europe for Subunit / Viral-like-Particle (VLP) Vaccines, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040)
- 24.1.2. RSV Vaccine Market in Europe for Live Attenuated Vaccines, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040)
- 24.1.3. RSV Vaccine Market in Europe for mRNA Vaccines, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040)
- 24.2. RSV Vaccine Market in Europe: Distribution by Route of Administration
- 24.2.1. RSV Vaccine Market in Europe for Intramuscular, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040)
- 24.2.2. RSV Vaccine Market in Europe for Other Routes, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040)
- 24.3. RSV Vaccine Market in Europe: Distribution by Target Patient Population
- 24.3.1. RSV Vaccine Market in Europe for Elderly Individuals, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040)
- 24.3.2. RSV Vaccine Market in Europe for Infants / Children, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040)
- 24.3.3. RSV Vaccine Market in Europe for Pregnant Individuals, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040)
- 24.4. RSV Vaccine Market in Europe: Distribution by Distribution Channel
- 24.4.1. RSV Vaccine Market in Europe for Pharmacies and Drug Stores, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040)
- 24.4.2. RSV Vaccine Market in Europe for Government / Institutional Suppliers, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040)
- 24.4.3. RSV Vaccine Market in Europe for Others, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040)
- *Detailed information on Section VII is available in the Excel Data Packs shared along with the report**
SECTION VIII: OTHER EXCLUSIVE INSIGHTS
25. CONCLUSION
26. EXECUTIVE INSIGHTS
SECTION IX: APPENDIX
27. TABULATED DATA
28. LIST OF COMPANIES AND ORGANIZATIONS
List of Tables
- Table 7.1 RSV Vaccines: Information on Developer, Stage of Development, Route of Administration and Type of Vaccine
- Table 7.2 RSV Vaccines: Information on Type of Protein Targeted, Type of Immunization and Target Patient Population
- Table 7.3 RSV Vaccines: List of Developers
- Table 9.1 Icosavax (A Company of AstraZeneca): Product Portfolio
- Table 9.2 Moderna: Product Portfolio
- Table 9.3 Pfizer: Product Portfolio
- Table 10.1 GlaxoSmithKline: Product Portfolio
- Table 10.2 Sanofi: Product Portfolio
- Table 11.1 Beijing Advaccine Biotechnology: Product Portfolio
- Table 13.1 RSV Vaccine Market: List of Published Patents
- Table 23.1 RSV Vaccine Market in North America: Distribution by Type of Vaccine, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040) (USD Million)
- Table 23.2 RSV Vaccine Market in North America: Distribution by Route of Administration, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040) (USD Million)
- Table 23.3 RSV Vaccine Market in North America: Distribution by Target Patient Population, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040) (USD Million)
- Table 23.4 RSV Vaccine Market in North America: Distribution by Distribution Channel, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040) (USD Million)
- Table 24.1 RSV Vaccine Market in Europe: Distribution by Type of Vaccine, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040) (USD Million)
- Table 24.2 RSV Vaccine Market in Europe: Distribution by Route of Administration, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040) (USD Million)
- Table 24.3 RSV Vaccine Market in Europe: Distribution by Target Patient Population, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040) (USD Million)
- Table 24.4 RSV Vaccine Market in Europe: Distribution by Distribution Channel, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040) (USD Million)
- *Detailed information on Tables 23.1-24.4 is available in the Excel Data Packs shared along with the report
- Table 27.1 RSV Vaccines: Distribution by Stage of Development
- Table 27.2 RSV Vaccines: Distribution by Route of Administration
- Table 27.3 RSV Vaccines: Distribution by Type of Vaccine
- Table 27.4 RSV Vaccines: Distribution by Type of Protein Targeted
- Table 27.5 RSV Vaccines: Distribution by Type of Immunization
- Table 27.6 RSV Vaccines: Distribution by Target Patient Population
- Table 27.7 RSV Vaccine Developers: Distribution by Year of Establishment
- Table 27.8 RSV Vaccine Developers: Distribution by Company Size
- Table 27.9 RSV Vaccine Developers: Distribution by Location of Headquarters
- Table 27.10 Moderna: Consolidated Financial Details, FY 2021 onwards (USD Million)
- Table 27.11 Pfizer: Business Segment-wise Revenues and Consolidated Financial Details, FY 2021 onwards (USD Million)
- Table 27.12 GlaxoSmithKline: Business Segment-wise Revenues and Consolidated Financial Details, FY 2021 onwards (EUR Million)
- Table 27.13 Sanofi: Business Segment-wise Revenues and Consolidated Financial Details, FY 2021 onwards (EUR Million)
- Table 27.14 Clinical Trial Analysis: Distribution by Trial Registration Year, Since 2015
- Table 27.15 Clinical Trial Analysis: Distribution by Trial Status
- Table 27.16 Clinical Trial Analysis: Distribution of Enrolled Patient Population by Trial Registration Year, Since 2015
- Table 27.17 Clinical Trial Analysis: Distribution by Trial Registration Year and Trial Status, Since 2015
- Table 27.18 Clinical Trial Analysis: Distribution by Trial Phase
- Table 27.19 Clinical Trial Analysis: Distribution of Enrolled Patient Population by Trial Phase
- Table 27.20 Clinical Trial Analysis: Distribution by Study Design
- Table 27.21 Clinical Trial Analysis: Distribution by Type of Sponsor / Collaborator
- Table 27.22 Clinical Trial Analysis: Distribution by Gender
- Table 27.23 Clinical Trial Analysis: Distribution by Target Patient Population
- Table 27.24 Leading Players: Distribution by Number of Registered Trial
- Table 27.25 Clinical Trial Analysis: Distribution of Clinical Trials by Geography
- Table 27.26 Clinical Trial Analysis: Distribution by Trial Status and Geography
- Table 27.27 Clinical Trial Analysis: Distribution of Enrolled Patient Population by Trial Status and Geography
- Table 27.28 Patent Analysis: Distribution by Patent Publication Year, Since 2020
- Table 27.29 Patent Analysis: Distribution by Type of Patent
- Table 27.30 Patent Analysis: Distribution by Type of Patent and Publication Year, Since 2020
- Table 27.31 Patent Analysis: Distribution by Patent Application Year, Since 2008
- Table 27.32 Patent Analysis: Distribution by Patent Age
- Table 27.33 Cumulative Year-wise Distribution by Type of Applicant
- Table 27.34 Patent Analysis: Distribution by Patent Jurisdiction
- Table 27.35 Leading Industry Players: Distribution by Number of Patents
- Table 27.36 Leading Non-Industry Players: Distribution by Number of Patents
- Table 27.37 Leading Inventors: Distribution by Number of Patents
- Table 27.38 RSV Vaccines: Patent Valuation
- Table 27.39 Global RSV Vaccine Market, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040) (USD Million)
- Table 27.40 Global RSV Vaccine Market, Forecasted Estimates (Till 2040): Conservative Scenario (USD Million)
- Table 27.41 Global RSV Vaccine Market, Forecasted Estimates (Till 2040): Optimistic Scenario (USD Million)
- Table 27.42 RSV Vaccine Market: Distribution by Type of Vaccine
- Table 27.43 RSV Vaccine Market for Subunit / Viral-like-Particle (VLP) Vaccines, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040): Conservative, Base and Optimistic Scenario (USD Million)
- Table 27.44 RSV Vaccine Market for Live Attenuated / Chimeric Vaccines, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040): Conservative, Base and Optimistic Scenario (USD Million)
- Table 27.45 RSV Vaccine Market for mRNA Vaccines, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040): Conservative, Base and Optimistic Scenario (USD Million)
- Table 27.46 RSV Vaccine Market: Distribution by Route of Administration
- Table 27.47 RSV Vaccine Market for Intramuscular, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040): Conservative, Base and Optimistic Scenario (USD Million)
- Table 27.48 RSV Vaccine Market for Other Routes, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040): Conservative, Base and Optimistic Scenario (USD Million)
- Table 27.49 RSV Vaccine Market: Distribution by Target Patient Population
- Table 27.50 RSV Vaccine Market for Elderly Individuals, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040): Conservative, Base and Optimistic Scenario (USD Million)
- Table 27.51 RSV Vaccine Market for Infants / Children, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040): Conservative, Base and Optimistic Scenario (USD Million)
- Table 27.52 RSV Vaccine Market for Pregnant Individuals, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040): Conservative, Base and Optimistic Scenario (USD Million)
- Table 27.53 RSV Vaccine Market: Distribution by Distribution channel
- Table 27.54 RSV Vaccine Market for Pharmacies and Drug Stores, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040): Conservative, Base and Optimistic Scenario (USD Million)
- Table 27.55 RSV Vaccine Market for Government / Institutional Suppliers, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040): Conservative, Base and Optimistic Scenario (USD Million)
- Table 27.56 RSV Vaccine Market for Others, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040): Conservative, Base and Optimistic Scenario (USD Million)
- Table 27.57 RSV Vaccine Market: Distribution by Key Geographical Regions
- Table 27.58 RSV Vaccine Market in North America, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040): Conservative, Base and Optimistic Scenario (USD Million)
- Table 27.59 RSV Vaccine Market in Europe, Historical Trends (Since 2023) and Forecasted Estimates (Till 2040): Conservative, Base and Optimistic Scenario (USD Million)
- Table 27.60 Market Movement Analysis: Key Geographical Regions
- Table 27.61 Penetration Growth Matrix: Key Geographical Regions
- Table 27.62 RSV Vaccine Market: Distribution by Leading Players










