 |
市场调查报告书
商品编码
1737059
临床试验软体市场:各部署类型,提供类别,各软体功能,各终端用户,各地区Clinical Trial Software Market Distribution by Type of Deployment, Type of Delivery, Features of Software and Geographical Regions |
||||||
预计到 2035 年,全球临床试验软体市场规模将从目前的 6.9 亿美元增长至 36.8 亿美元,预测期内的复合年增长率为 14%。
市场区隔根据以下参数对市场规模和机会进行分类:
各部署类型
- 开云端
- 内部部署
提供类别
- 网站为基础的
- 远端监控
各软体功能
- EDC
- eCOA/ePRO
- eConsent
各终端用户
- 製药/生物科技产业
- 学术·研究机关
- 其他的产业
各地区
- 北美
- 欧洲
- 亚太地区
临床试验软体市场:成长和趋势
临床试验是前瞻性的生物医学研究,旨在评估人体内的医疗、外科和行为干预措施,并探索诊断和预防/治疗疾病的新方法。为了获得新型治疗干预措施的上市监管批准,需要高度准确且复杂的临床试验数据来验证药物针对特定适应症的安全性和有效性。然而,这种传统的临床研究方法面临着许多课题,包括高昂的资本投入、较低的患者采用率以及参与各种治疗干预措施开发的公司缺乏可靠的数据。这导致无法有效率地产生充足的临床证据,为药物开发公司以及获得救命治疗的患者带来了巨大的资金损失。事实上,传统的手动临床试验几乎占据了药物开发过程中50%的时间。在此背景下,临床试验软体因其能够即时分析、数据管理和追踪药物不良反应而备受关注。
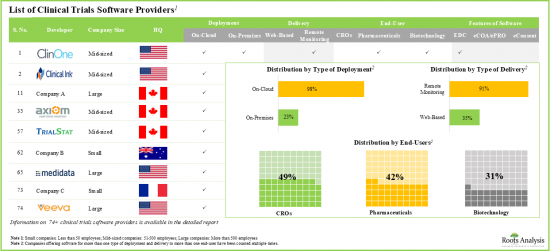
近年来,各公司开发了许多临床试验软体/临床试验管理系统,包括EDC、eCOA/ePRO和eConsent。这些工具支援即时临床试验,并提高患者对药物检测和分析的依从性。由于其重要性,许多临床试验软体市场参与者正在进入该领域并开发创新技术。值得注意的是,这些技术有助于提升临床试验结果,并减轻製药公司的负担。由于对自动化临床试验软体的需求不断增长,预计市场在预测期内将大幅增长。
临床试验软体市场:关键洞察
本报告深入探讨了临床试验软体市场的现状,并识别了行业内的潜在成长机会。主要发现包括:
- 全球超过 70 家公司声称已开发出能够实现临床研究方法分散化的软体解决方案,从而优化临床试验的时间和成本。
- 目前的市场格局高度分散,现有企业和新进业者都提供具有先进功能的临床试验软体。事实上,自 2000 年以来,已有超过 51 家专注于开发临床试验软体的新创公司成立。
- 为了建立竞争优势,各公司正积极扩展现有能力,增强各自的产品线,并紧跟不断变化的产业基准。
- 由于预期获得丰厚回报,许多公私投资者纷纷大举投资,促使融资活动激增。尤其是北美,该领域融资案例约占90%。
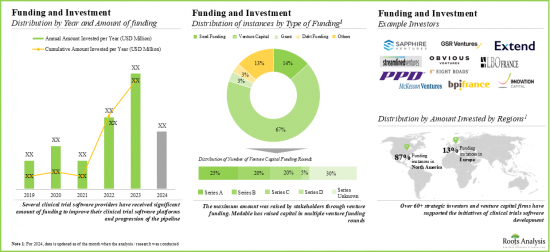
- 过去几年,该领域的合作伙伴关係和协作显着增加,显示利害关係人的兴趣日益浓厚。
- 值得注意的是,大多数併购案例发生在2023年,显示该领域正逐渐转向整合。此外,大多数交易(87%)是收购,其次是合併。
- 预计未来十年该市场将以每年14%的速度成长。机会可能因部署类型、软体功能、产品类型和地理区域而异。
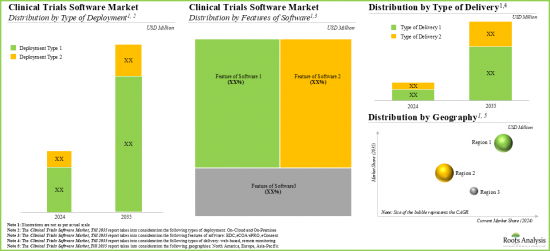
临床试验软体市场:关键细分市场
电子同意书 (eConsent) 软体细分市场占据全球临床试验软体市场的最大占有率
依软体功能划分,全球临床试验软体市场分为 EDC、eCOA/ePRO 和 eConsent 软体。目前,eConsent 软体占据临床试验软体市场的绝大部分占有率。值得注意的是,eCOA/ePRO 细分市场的全球临床试验软体市场很可能会以相对较高的复合年增长率成长。
依地区划分,市场分为北美、欧洲和亚太地区。目前来看,北美占据最大市场占有率,而亚太地区市场预计在预测期内将呈现良好成长。
本报告提供全球临床试验软体市场相关调查,提供市场概要,以及各部署类型,提供类别,各软体功能,各终端用户,各地区的趋势,及加入此市场的主要企业简介等资讯。
目录
第1章 序文
第2章 摘要整理
第3章 简介
- 章概要
- 临床研究的现有的规定
- 虚拟临床试验
- 虚拟临床试验管理相关的机会与课题
- 未来展望
第4章 市场形势:临床试验软体市场
- 章概要
- 临床试验软体市场:产品清单
- 临床试验软体市场:开发商的形势
第5章 北美的临床试验软体开发公司:企业简介
- 章概要
- Advarra
- Arisglobal
- AssistRx
- Clario
- IBM
- IQVIA
- Medidata
- Oracle
- Signant Health
- Veeva
第6章 欧洲的临床试验软体开发企业:企业简介
- 章概要
- Calyx
第7章 企业竞争力分析
第8章 伙伴关係和合作
第9章 合併和收购
第10章 与资金筹措投资分析
第11章 与市场预测机会分析
- 章概要
- 预测调查手法主要的前提条件
- 全球临床试验软体市场,2021年~2035年
- 临床试验软体市场,2021年~2035年:各部署类型分布
- 临床试验软体市场,2021年~2035年:提供类别分布
- 临床试验软体市场,2021年~2035年:各软体功能分布
- 临床试验软体市场,2021年~2035年:各地区分布
第12章 结论
第13章 附录1:表格形式的资料
第14章 附录2:企业·团体一览
CLINICAL TRIAL SOFTWARE MARKET: OVERVIEW
As per Roots Analysis, the global clinical trial software market is estimated to grow from USD 0.69 billion in the current year to USD 3.68 billion by 2035, at a CAGR of 14% during the forecast period, till 2035.
The market sizing and opportunity analysis has been segmented across the following parameters:
Type of Deployment
- On-Cloud
- On-Premises
Type of Delivery
- Web-Based
- Remote Monitoring
Features of Software
- EDC
- eCOA/ePRO
- eConsent
End Users
- Pharmaceutical / Biotechnology Industries
- Academic and Research Institutes
- Other Industries
Geographical Regions
- North America
- Europe
- Asia-Pacific
CLINICAL TRIAL SOFTWARE MARKET: GROWTH AND TRENDS
Clinical trials are prospective biomedical research studies designed to evaluate medical, surgical or behavioral interventions in people and investigate novel approaches for the diagnosis and prevention / treatment of diseases. In order to gain marketing approval from regulatory authorities for a novel therapeutic intervention, highly accurate and elaborate clinical trial data is required to validate the drug's safety and effectiveness towards a specific target indication. However, there are several challenges associated with this traditional way of clinical research. Some of these include high capital investment, low patient recruitment rates and lack of robust data faced by companies involved in the development of various therapeutic interventions. This leads to inefficiency in generating sufficient clinical evidence, resulting in massive capital losses for drug developers, as well as patients accessing these life-saving therapies. In fact, manual conventional clinical trials consume almost 50% of the time during drug development. In this context, clinical trial software has gained significant attention owing to its ability for real-time analysis, data management, and tracking of the adverse impact of drugs.

In recent years, various players have developed a number of clinical trial software / clinical trial management systems, including EDC, eCOA / ePRO, and eConsent. These tools enable real-time clinical studies and improve patient compliance for drug testing and analysis. Owing to its significance, several clinical trial software market players are currently engaged in this field to develop innovative technologies. Notably, these technologies encourage successful outcomes of clinical trials and reduce the burden of pharmaceutical companies. Given the increasing demand for automated clinical trial software, the market is expected to witness substantial growth during the forecast period.
CLINICAL TRIAL SOFTWARE MARKET: KEY INSIGHTS
The report delves into the current state of the clinical trial software market and identifies potential growth opportunities within the industry. Some key findings from the report include:
- 70+ companies worldwide claim to have developed software solutions allowing decentralization of the clinical research process, optimizing time and cost spent on clinical trials.
- The current market landscape is highly fragmented, with the presence of both established players and new entrants offering clinical trials software with advanced features. In fact, since 2000, over 51 start-ups focused on developing clinical trials software have been established.
- In pursuit of building a competitive edge, companies are actively expanding their existing capabilities to enhance their respective offerings and comply with evolving industry benchmarks.
- Foreseeing lucrative returns, many public and private investors have significant investments, marking a surge in funding activity. Notably, North America witnessed around 90% of funding instances in this domain.

- The domain has witnessed a considerable increase in partnerships and collaborations over the past few years, indicating the rising interest of stakeholders.
- Notably, most of the M&A instances were reported in 2023, indicating a gradual shift towards consolidation. Further, majority (87%) of agreements were acquisitions, followed by mergers.
- We expect the market to grow at an annualized rate of 14% in the coming decade; the opportunity is likely to be well distributed across type of deployment, features of software, type of delivery and geographical regions.

CLINICAL TRIAL SOFTWARE MARKET: KEY SEGMENTS
The eConsent Software Segment Holds the Largest Share of the Global Clinical Trial Software Market
Based on the features of software, the global market for clinical trial software is segmented into EDC, eCOA/ePRO and eConsent software. Currently, the majority of the clinical trial software market is captured by eConsent software. It is worth highlighting that the global clinical trial software market for eCOA / ePRO segment is likely to grow at a relatively higher CAGR.
North America Accounts for the Largest Share of the Market
Based on the geographical regions, the market is segmented into North America, Europe and Asia-Pacific. In the current scenario, North America is likely to capture the largest market share while the market in Asia-Pacific is anticipated to demonstrate lucrative growth during the forecast period.
Example Players in the Clinical Trial Software Market
- Advarra
- Arisglobal
- AssistRx
- Calyx
- Clario
- IBM
- IQVIA
- Medidata
- Oracle
- Signant Health
- Veeva
CLINICAL TRIAL SOFTWARE MARKET: RESEARCH COVERAGE
- Market Sizing and Opportunity Analysis: The report features an in-depth analysis of the global clinical trial software market, focusing on key market segments, including [A] type of deployment, [B] type of delivery, [C] features of software, [D] end users and [E] geographical regions.
- Clinical Trials Software Market Landscape: A comprehensive evaluation of clinical trials software market, based on several relevant parameters, such as [A] type of deployment, [B] type of delivery, [C] type of end-user, [D] features of software, [E] trial design and [F] type of technology. Additionally, a comprehensive evaluation of the companies engaged in developing clinical trials software, based on several relevant parameters, such as [G] year of establishment, [H] company and [I] location of headquarters.
- Company Profiles: In-depth profiles of key players engaged in the development of clinical trials software, focusing on [A] overview of the company, [B] product portfolio, and [C] recent developments and [D] an informed future outlook.
- Company Competitiveness Analysis: A comprehensive competitive analysis of clinical trials software developers, examining factors, such as [A] supplier strength, [B] product portfolio strength and [C] service applicability.
- Partnerships and Collaborations: An insightful analysis of the deals inked by stakeholders in the global clinical trials software market, based on several parameters, such as [A] year of partnership, [B] type of partnership, [C] geographical distribution of partnership activity and [D] most active players (in terms of the number of partnerships signed).
- Mergers and Acquisitions: An in-depth analysis of the mergers and acquisitions undertaken in this domain, based on relevant parameters, such as [A] type of agreement, [B] year of mergers and acquisitions, [C] geographical location and [D] most active players (in terms of the number of mergers and acquisitions).
- Funding and Investment Analysis: An in-depth analysis of the fundings raised by companies engaged in this domain, based on relevant parameters, such as [A] year of funding, [B] type of funding, [C] amount invested, [D] most active players and [F] most active investors.
KEY QUESTIONS ANSWERED IN THIS REPORT
- How many companies are currently engaged in this market?
- Which are the leading companies in this market?
- What factors are likely to influence the evolution of this market?
- What is the current and future market size?
- What is the CAGR of this market?
- How is the current and future market opportunity likely to be distributed across key market segments?
REASONS TO BUY THIS REPORT
- The report provides a comprehensive market analysis, offering detailed revenue projections of the overall market and its specific sub-segments. This information is valuable to both established market leaders and emerging entrants.
- Stakeholders can leverage the report to gain a deeper understanding of the competitive dynamics within the market. By analyzing the competitive landscape, businesses can make informed decisions to optimize their market positioning and develop effective go-to-market strategies.
- The report offers stakeholders a comprehensive overview of the market, including key drivers, barriers, opportunities, and challenges. This information empowers stakeholders to stay abreast of market trends and make data-driven decisions to capitalize on growth prospects.
ADDITIONAL BENEFITS
- Complimentary PPT Insights Packs
- Complimentary Excel Data Packs for all Analytical Modules in the Report
- 15% Free Content Customization
- Detailed Report Walkthrough Session with Research Team
- Free Updated report if the report is 6-12 months old or older
TABLE OF CONTENTS
1. PREFACE
- 1.1. Scope of the Report
- 1.2. Research Methodology
- 1.3. Key Questions Answered
- 1.4. Chapter Outlines
2. EXECUTIVE SUMMARY
3. INTRODUCTION
- 3.1. Chapter Overview
- 3.2. Existing Constraints in Clinical Research
- 3.2.1. Increasing Trial Costs and Complexity
- 3.2.2. Evolving Regulatory Standards
- 3.2.3. Patient Recruitment and Retention-Related Challenges
- 3.2.4. Inefficient Data Handling
- 3.3. Virtual Clinical Trials
- 3.3.1. Electronic Data Capture Solutions
- 3.3.2. Electronic Clinical Outcome Assessment and Electronic Patient Reported Outcome Solutions (eCOA / ePRO)
- 3.3.3. Electronic Consent Solutions
- 3.4. Opportunities and challenges associated with Virtual Clinical Trials Management
- 3.5 Future Perspectives
4 MARKET LANDSCAPE: CLINICAL TRIALS SOFTWARE MARKET
- 4.1. Chapter Overview
- 4.2. Clinical Trials Software Market: List of Products
- 4.2.1. Analysis by Type of Deployment
- 4.2.2. Analysis by Type of Delivery
- 4.2.3. Analysis by End-User
- 4.2.4. Analysis by Features of Software
- 4.2.5. Analysis by Trial Design
- 4.2.6. Analysis by Type of Technology
- 4.3. Clinical Trials Software Market: Developer Landscape
- 4.3.1. Analysis by Year of Establishment
- 4.3.2. Analysis by Company Size
- 4.3.3. Analysis by Geography
5 CLINICAL TRIALS SOFTWARE DEVELOPERS IN NORTH AMERICA: COMPANY PROFILES
- 5.1. Chapter Overview
- 5.2. Advarra
- 5.2.1. Company Overview
- 5.2.2. Product Portfolio: Clinical Trials Software
- 5.2.3. Recent Developments and Future Outlook
- 5.3. Arisglobal
- 5.3.1. Company Overview
- 5.3.2. Product Portfolio: Clinical Trials Software
- 5.3.3. Recent Developments and Future Outlook
- 5.4. AssistRx
- 5.4.1. Company Overview
- 5.4.2. Product Portfolio: Clinical Trials Software
- 5.4.3. Recent Developments and Future Outlook
- 5.5. Clario
- 5.5.1. Company Overview
- 5.5.2. Product Portfolio: Clinical Trials Software
- 5.5.3. Recent Developments and Future Outlook
- 5.6. IBM
- 5.6.1. Company Overview
- 5.6.2. Product Portfolio: Clinical Trials Software
- 5.6.3. Recent Developments and Future Outlook
- 5.7. IQVIA
- 5.7.1. Company Overview
- 5.7.2. Product Portfolio: Clinical Trials Software
- 5.7.3. Recent Developments and Future Outlook
- 5.8. Medidata
- 5.8.1. Company Overview
- 5.8.2. Product Portfolio: Clinical Trials Software
- 5.8.3. Recent Developments and Future Outlook
- 5.9. Oracle
- 5.9.1. Company Overview
- 5.9.2. Product Portfolio: Clinical Trials Software
- 5.9.3. Recent Developments and Future Outlook
- 5.10. Signant Health
- 5.10.1. Company Overview
- 5.10.2. Product Portfolio: Clinical Trials Software
- 5.10.3. Recent Developments and Future Outlook
- 5.11. Veeva
- 5.11.1. Company Overview
- 5.11.2. Product Portfolio: Clinical Trials Software
- 5.11.3. Recent Developments and Future Outlook
6 CLINICAL TRIALS SOFTWARE DEVELOPERS IN EUROPE: COMPANY PROFILES
- 6.1. Chapter overview
- 6.2. Calyx
- 6.2.1. Company Overview
- 6.2.2. Product Portfolio: Clinical Trials Software
- 6.2.3. Recent Developments and Future Outlook
7 COMPANY COMPETITIVENESS ANALYSIS
- 7.1. Chapter Overview
- 7.2. Key Parameters and Methodology
- 7.3. Competitiveness Analysis: Companies providing clinical trials software developers
- 7.4. Competitiveness Analysis: Companies providing clinical trials software in North America
- 7.5. Competitiveness Analysis: Companies providing clinical trials software in Europe
- 7.6. Competitiveness Analysis: Companies providing clinical trials software in Asia-Pacific
8 Partnerships and Collaborations
- 8.1. Chapter Overview
- 8.2. Partnership Models
- 8.3. Clinical Trials Software Market: Partnerships and Collaborations
- 8.3.1. Analysis by Year of Partnership
- 8.3.2. Analysis by Type of Partnership
- 8.3.3. Analysis by Type and Year of Partnership
- 8.4. Geographical Analysis
- 8.4.1. Analysis by Intracontinental and Intercontinental Agreements
- 8.4.2. Analysis by Local and International Agreements
- 8.5. Most Active Players: Analysis by Number of Partnerships
9 MERGERS AND ACQUISITIONS
- 9.1. Chapter Overview
- 9.2. Mergers and Acquisitions Models
- 9.3. Clinical Trials Software Market: Mergers and Acquisitions
- 9.3.1. Analysis by Type of Agreement
- 9.3.2. Analysis by Year of Mergers and Acquisitions
- 9.4. Analysis by Geographical Activity
- 9.4.1. Region-wise Analysis
- 9.4.2. Mergers and Acquisitions: Intercontinental and Intracontinental Deals
- 9.5. Most Active Players: Analysis by Number of Instances Acquisitions and Mergers
10 FUNDING AND INVESTMENT ANALYSIS
- 10.1. Chapter Overview
- 10.2. Types of Funding Instances
- 10.3. Clinical Trials Software Market: Recent Funding Instances
- 10.3.1. Analysis by Year of Investment
- 10.3.2. Analysis by Amount Invested
- 10.3.3. Analysis by Type of Funding
- 10.4. Most Active Players: Analysis by Number of Funding Instances
- 10.5. Regional Analysis by Amount Invested
- 10.6. Concluding Remarks
11 MARKET FORECAST AND OPPORTUNITY ANALYSIS
- 11.1. Chapter Overview
- 11.2. Forecast Methodology and Key Assumptions
- 11.3. Global Clinical Trials Software Market, 2021-2035
- 11.3.1. Clinical Trials Software Market, 2021-2035: Distribution by Type of Deployment
- 11.3.2. Clinical Trials Software Market, 2021-2035: Distribution by Type of Delivery
- 11.3.3. Clinical Trials Software Market, 2021-2035: Distribution by Features of Software
- 11.3.4. Clinical Trials Software Market, 2021-2035: Distribution by Geographical Region
- 11.3.4.1. Clinical Trials Software Market in North America, 2021-2035
- 11.3.4.1.1. Clinical Trials Software Market in North America, 2021-2035: Distribution by Type of Deployment
- 11.3.4.1.2. Clinical Trials Software Market in North America, 2021-2035: Distribution by Type of Delivery
- 11.3.4.1.3. Clinical Trials Software Market in North America, 2021-2035: Distribution by Features of Software
- 11.3.4.2. Clinical Trials Software Market in Europe, 2021-2035
- 11.3.4.2.1. Clinical Trials Software Market in Europe, 2021-2035: Distribution by Type of Deployment
- 11.3.4.2.2. Clinical Trials Software Market in Europe, 2021-2035: Distribution by Type of Delivery
- 11.3.4.2.3. Clinical Trials Software Market in Europe, 2021-2035: Distribution by Features of Software
- 11.3.4.3. Clinical Trials Software Market in Asia-Pacific, 2021-2035
- 11.3.4.3.1. Clinical Trials Software Market in Asia-Pacific, 2021-2035: Distribution by Type of Deployment
- 11.3.4.3.2. Clinical Trials Software Market Asia-Pacific, 2021-2035: Distribution by Type of Delivery
- 11.3.4.3.3. Clinical Trials Software Market Asia-Pacific, 2021-2035: Distribution by Features of Software
- 11.3.4.1. Clinical Trials Software Market in North America, 2021-2035
12. CONCLUSION
- 12.1. Chapter Overview
13. Appendix 1: Tabulated Data
14. Appendix 2: List of Companies and Organizations
List of Tables
- Table 4.1 Clinical Trials Software Market: Information on Type of Deployment, Type of Delivery, Type of End-User, Type of features of Software, Type of Trial Design and Type of Technology
- Table 4.2 Clinical Trials Software Market Developers: Information of Year of Establishment, Company Size and Location of Headquarters
- Table 4.3 Clinical Trials Software Market Developers: List of Software and Developers
- Table 6.1 Clinical Trials Software Market: List of Developers in North America
- Table 6.2 Advarra: Company Snapshot
- Table 6.3 Advarra: Product Portfolio
- Table 6.4 Advarra: Recent Developments and Future Outlook
- Table 6.5 ArisGlobal: Company Snapshot
- Table 6.6 ArisGlobal: Product Portfolio
- Table 6.7 ArisGlobal: Recent Developments and Future Outlook
- Table 6.8 AssistRx: Company Snapshot
- Table 6.9 AssistRx: Product Portfolio
- Table 6.10 AssistRx: Recent Developments and Future Outlook
- Table 6.11 Clario: Company Snapshot
- Table 6.12 Clario: Product Portfolio
- Table 6.13 Clario: Recent Developments and Future Outlook
- Table 6.14 IBM: Company Snapshot
- Table 6.15 IBM: Product Portfolio
- Table 6.16 IBM: Recent Developments and Future Outlook
- Table 6.17 IQVIA: Company Snapshot
- Table 6.18 IQVIA: Product Portfolio
- Table 6.19 IQVIA: Recent Developments and Future Outlook
- Table 6.20 Medidata: Company Snapshot
- Table 6.21 Medidata: Product Portfolio
- Table 6.22 Medidata: Recent Developments and Future Outlook
- Table 6.23 Oracle: Company Snapshot
- Table 6.24 Oracle: Product Portfolio
- Table 6.25 Oracle: Recent Developments and Future Outlook
- Table 6.26 Signant Health: Company Snapshot
- Table 6.27 Signant Health: Product Portfolio
- Table 6.28 Signant Health: Recent Developments and Future Outlook
- Table 6.29 Veeva: Company Snapshot
- Table 6.30 Veeva: Product Portfolio
- Table 6.31 Veeva: Recent Developments and Future Outlook
- Table 6.32 Oracle: Company Snapshot
- Table 6.33 Oracle: Product Portfolio
- Table 6.34 Oracle: Recent Developments and Future Outlook
- Table 7.1 Clinical Trials Software Market: List of developers in Europe
- Table 7.2 Calyx: Company Snapshot
- Table 7.3 Calyx: Product Portfolio
- Table 7.4 Calyx: Recent Developments and Future Outlook
- Table 9.1 Clinical Trials Software Market: List of Partnerships and Collaborations, 2016- 2021 (till September)
- Table 10.1 Clinical Trials Software Market: List of Mergers and Acquisitions, 2016-2021 (till September)
- Table 11.1 Clinical Trials Software Market: List of Funding Instances, 2016-2021 (till September)
List of Figures
- Figure 2.1 Executive Summary: Current Market Landscape of Clinical Trials Software Market
- Figure 2.2 Executive Summary: Partnerships and Collaborations
- Figure 2.3 Executive Summary: Funding and Investment
- Figure 2.4 Executive Summary: Market Forecast and Opportunity Analysis
- Figure 4.1 Clinical Trials Software: Distribution by Type of Deployment
- Figure 4.2 Clinical Trials Software: Distribution by Type of Delivery
- Figure 4.3 Clinical Trials Software: Distribution by Type of End-User
- Figure 4.4 Clinical Trials Software: Distribution by Features of Software
- Figure 4.5 Clinical Trials Software: Distribution by Trial Design
- Figure 4.6 Clinical Trials Software: Distribution by Type of Technology
- Figure 4.7 Clinical Trials Software Developers: Distribution by Year of Establishment
- Figure 4.8 Clinical Trials Software Developers: Distribution by Company Size
- Figure 4.9 Clinical Trials Software Developers: Distribution by Geography
- Figure 7.1 Company Competitiveness Analysis: Clinical Trials Software Developers in North America
- Figure 7.2 Company Competitiveness Analysis: Clinical Trials Software Developers in Europe
- Figure 7.3 Company Competitiveness Analysis: Clinical Trials Software Developers in Asia Pacific
- Figure 8.1 Partnerships and Collaborations: Distribution by Year of Partnership
- Figure 8.2 Partnerships and Collaborations: Distribution by Type of Partnership
- Figure 8.3 Partnerships and Collaborations: Distribution by Year and Type of Partnership
- Figure 8.4 Partnerships and Collaborations: Geographical Analysis
- Figure 8.5 Partnerships and Collaborations: Distribution by Intracontinental and Intercontinental Agreement
- Figure 8.6 Most Active Players: Distribution by Number of Partnerships
- Figure 9.1 Mergers and Acquisitions: Distribution by Type of Merger and Acquisition
- Figure 9.2 Mergers and Acquisitions: Distribution by Year of Merger and Acquisition
- Figure 9.3 Mergers and Acquisitions: Distribution by Geographical Activity
- Figure 9.4 Mergers and Acquisitions: Continent-wise Distribution
- Figure 9.5 Mergers and Acquisitions: Intercontinental and Intracontinental Deals
- Figure 9.6 Most Active Players: Distribution by Number of Instances
- Figure 10.1 Funding and Investments: Distribution by Year of Investment
- Figure 10.2 Funding and Investments: Distribution by Amount Invested
- Figure 10.3 Funding and Investments: Distribution by Year-wise Trend of Amount Invested, 2016-2021
- Figure 10.4 Funding and Investments: Distribution by Type of Funding
- Figure 10.5 Funding and Investments: Distribution of Amount Invested by Type of Funding
- Figure 10.6 Most Active Players: Distribution by Amount invested
- Figure 10.7 Funding and Investments: Geographical Distribution by Amount Invested, 2016-2021
- Figure 11.1 Clinical Trials Software Market, 2021-2035 (USD Million)
- Figure 11.2 Clinical Trials Software Market, 2021-2035: Distribution by Type of Deployment (USD Million)
- Figure 11.3 Clinical Trials Software Market, 2021-2035: Distribution by Type of Delivery (USD Million)
- Figure 11.4 Clinical Trials Software Market, 2021-2035: Distribution by Features of Software (USD Million)






