 |
市场调查报告书
商品编码
1771303
细胞重编程市场:产业趋势及全球预测 - 依技术类型、细胞来源类型、应用类型和主要地区Cell Reprogramming Market: Industry Trends and Global Forecasts - Distribution by Type of Technology, Type of Source Cell, Type of Application and Key Geographical Regions |
||||||
全球细胞重编程市场:概览
今年全球细胞重编程市场规模达 11.6亿美元。预计预测期内,该市场的年复合成长率将达到 8%。
市场区隔与机会分析依下列参数划分:
技术类型
- 基于仙台病毒的重编程
- mRNA重编程
- 游离型重编程
- 其他重编程技术
细胞来源类型
- 纤维母细胞
- 週边血单核细胞
- 未指定的体细胞
- 其他
应用类型
- 研究
- 治疗
主要地区
- 北美
- 欧洲
- 亚太地区及世界其他地区
全球细胞重编程市场:成长与趋势
干细胞研究进展多年来,细胞生物学和再生医学的发展促成了各种基于干细胞的疗法的发现和发展。然而,细胞疗法的开发和应用仍然存在挑战,包括复杂的製造流程、健康细胞捐赠者的短缺以及与捐赠者-接受者单倍型错配相关的担忧。此外,将人类干细胞用于研究目的也存在一些伦理障碍。因此,细胞重编程的概念应运而生,成为克服上述问题并发展安全有效的细胞治疗介入的手段之一。
在生物技术领域的技术发展和再生医学相关市场发展的推动下,全球许多研究小组目前开发创新的细胞重编程方法。此外,值得注意的是,各类新创公司和大学衍生公司已成为这一新兴治疗领域的先驱,预计将在未来几年保持研究动力。
全球细胞重编程市场:关键洞察
本报告深入探讨了全球细胞重编程市场的现状,并识别了产业内的潜在成长机会。报告的主要调查结果包括:
- 细胞重编程技术的进步为许多先进的治疗和研究应用铺平了道路,为行业和非行业服务提供商公司带来了丰厚的商机。
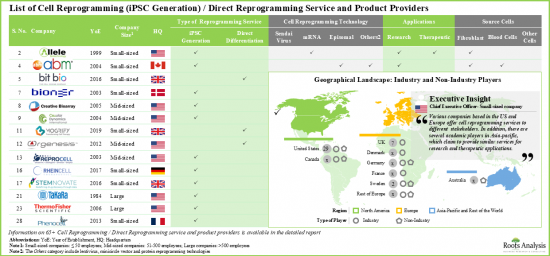
- 超过 30 家公司声称提供细胞重编程服务和产品。其中近 70%是小型企业(员工人数少于 50 人)。
- 目前,大多数服务提供者(约 75%)使用游离重编程技术提供 iPSC 生成服务,并声称能够处理多种细胞类型。其次是提供直接重编程服务的公司(30%)。
- 值得注意的是,大多数利害关係人声称使用游离重编程方法来产生iPSC。其他流行的细胞重编程方法包括仙台病毒和基于mRNA的技术。
- 许多公司声称使用多种技术来产生iPS细胞。例如Applied StemCell、Applied Biological Materials、Creative Bioarray、Lonza和Stemnovate。
- 考虑到纤维母细胞的各种优势,例如其易于获取、增殖速度快和细胞稳健性,这些细胞在细胞重编程中得到了最大程度的利用(超过40%)。
- 细胞重编程领域以众多中小型公司为特征,分布在各个地区,参与者建立了多个设施来满足客户的需求。
- 北美是领先的细胞重编程服务中心。超过55%参与细胞重编程的公司总部位于北美,其次是总部位于欧洲的公司(45%)。
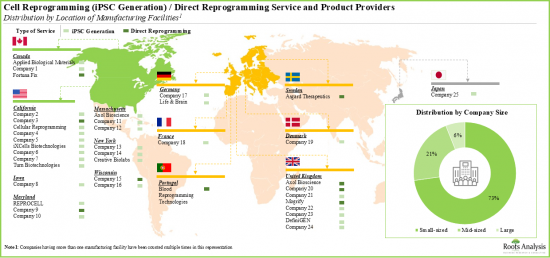
- 欧洲领先的机构包括英国、德国、丹麦、瑞士、法国、葡萄牙和瑞典。各利益相关者正积极扩展其产能,以丰富其细胞重编程服务组合,并在未来保持产业竞争力。
- 过去达成的包括各种细胞类型的合作数量反映了人们对该领域日益成长的兴趣。
- 自2016年以来,合作活动以7%的年复合成长率成长。随着对干细胞的需求不断成长,产业界和学术界的参与者很可能会就细胞重编程建立多种合作关係。
- 过去几年,现有企业和新进业者都已建立多项策略伙伴关係。大多数交易(超过30%)主要用于iPS细胞生成,并根据客户需求进一步细分。
- 许多公司已签署多项细胞重编程协议。签署四份或更多协议的公司包括Cellaria、BlueRock Therapeutics、FUJIFILM Cellular Dynamics和STEMCELL Technologies。
- 过去几年中,已有540多项临床试验启动,评估各种类型的干细胞疗法,凸显了对iPS细胞生成和直接重编程策略日益成长的需求。
- 超过70%的临床试验由中小企业赞助,目的是探索干细胞疗法的潜力。
- 一项针对300多家参与干细胞疗法开发的利害关係人的调查显示,不同地区的公司可以成为细胞重编程服务提供者的策略伙伴。
- 预计到2030年,该市场的年复合成长率将达到8%,机会可能遍布各种技术类型、应用领域和主要地区。
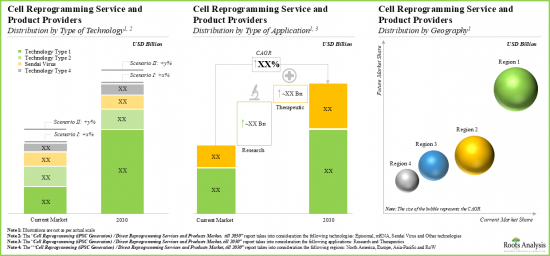
- 目前,大部分服务收入来自包括游离型重编程技术的计画。
- 预计到2030年,致力于游离型重编程的计画将占到总收入的40%以上,其次是基于仙台病毒的重编程计画(30%)和mRNA重编程计画(25%)的收入。
细胞重编程市场参与者
- Allele Biotechnology
- ALSTEM
- Applied Biological Materials
- Axol Bioscience
- Creative Bioarray
- DefiniGEN
- FUJIFILM Cellular Dynamics
- Lonza
- Mogrify
- REPROCELL
- Stemnovate
- Thermo Fisher Scientific
本报告调查全球细胞重编程市场,提供市场概述,以及依技术类型、细胞来源类型、应用类型和地区的趋势,和参与市场的公司简介。
目录
第1章 简介
第2章 执行摘要
第3章 简介
- 章节概述
- 干细胞概述
- 细胞重编程简介
- 细胞重编程的应用
- 主要成长与限制因素
第4章 当前市场格局
- 章节概述
- 细胞重编程服务与产品提供者:产业参与者列表
- 细胞重编程服务与产品提供者:非产业参与者列表
- 细胞重编程:相关产品提供者列表
章节5:公司竞争力分析
- 章节概述
- 假设和关键参数
- 研究方法
- 竞争分析:iPSC 产生服务与产品供应商
- 竞争分析:直接重编程服务与产品供应商
第6章 公司简介
- 章节概述
- Allele Biotechnology
- ALSTEM
- Applied Biological Materials
- Axol Bioscience
- Creative Bioarray
- DefiniGEN
- FUJIFILM Cellular Dynamics
- Lonza
- Mogrify
- REPROCELL
- Stemnovate
- Thermo Fisher Scientific
第7章 案例研究:干细胞疗法研发中的临床试验活动
- 章节概述
- 研究范围与研究方法
- 干细胞疗法:临床试验分析
第8章 合作伙伴与合作
- 章节概述
- 研究范围与研究方法
- 细胞重编程服务与产品市场:合作伙伴与合作清单
第9章 合作伙伴潜力
- 章节概述
- 研究范围与研究方法
- 评分标准与关键假设
- 干细胞疗法开发商:细胞重编程服务和产品提供者的有前途的合作伙伴
- 干细胞疗法合约製造商:细胞重编程服务和产品提供者的有前途的合作伙伴
第10章 市场预测
- 章节概述
- 范围与研究方法
- 预测研究方法与关键假设
- 2035年全球细胞重编程服务与市场占有率及产品市场
- 全球细胞重编程服务与产品市场(依技术类型)(2035)
- 全球细胞重编程服务与产品市场(依细胞来源类型)(2035)
- 全球细胞重编程服务与产品市场(依应用类型)(2035)
- 全球细胞重编程服务与产品市场(依主要地区)(2035)
第11章 高层洞察
第12章 结论
第13章 附录1:表格资料
第14章 附录2:公司与组织清单
GLOBAL CELL REPROGRAMMING MARKET: OVERVIEW
As per Roots Analysis, the global cell reprogramming market valued at USD 1.16 billion in the current year is anticipated to grow at a CAGR of 8% during the forecast period.
The market sizing and opportunity analysis has been segmented across the following parameters:
Type of Technology
- Sendai Virus-based Reprogramming
- mRNA Reprogramming
- Episomal Reprogramming
- Other Reprogramming Technologies
Type of Source Cell
- Fibroblasts
- Peripheral Blood Mononuclear Cells
- Unspecified Somatic Cells
- Other Cells
Type of Application
- Research
- Therapeutic
Key Geographical Regions
- North America
- Europe
- Asia-Pacific and Rest of the World
GLOBAL CELL REPROGRAMMING MARKET: GROWTH AND TRENDS
Progress in stem cell biology and regenerative medicine over the years has led to the discovery and development of various stem cell-based therapies. However, prevalent challenges concerning the development and use of cell therapies persist, including complex production process, scarcity of healthy cell donors, and concerns associated with donor-recipient haplotype mismatch. Moreover, there are still several ethical barriers when it comes to using human stem cells for research purposes. Consequently, the concept of cell reprogramming has emerged as one of the means to overcome the aforementioned issues and develop safe and effective cell-based therapeutic interventions.

Empowered by technical developments in the field of biotechnology and the market opportunity associated with regenerative medicine, a number of research groups across the world are presently developing innovative ways to reprogram cells. Further, it is worth noting that various start-ups and university spin-offs have emerged as pioneers in this emerging field of therapeutics and are anticipated to maintain the research momentum in the coming years as well.
GLOBAL CELL REPROGRAMMING MARKET: KEY INSIGHTS
The report delves into the current state of global cell reprogramming market and identifies potential growth opportunities within industry. Some key findings from the report include:
- Advances in cell reprogramming have paved way for a plethora of advanced therapeutic and research applications, resulting in lucrative business opportunities for industry and non-industry service provider firms.
- More than 30 companies claim to offer cell reprogramming services and products. Of these, close to 70% are small firms (having less than 50 employees).
- Presently, the majority (~75%) of the service providers claim to offer iPSC generation services, using episomal reprogramming technologies, and are capable of working with various types of cells. This is followed by the companies offering direct reprogramming services (30%).
- It is worth mentioning that majority of stakeholders claim to be using episomal reprogramming for generating iPSCs; other popular cell reprograming approaches include Sendai virus and mRNA-based technologies.
- Numerous companies claim to use more than two technologies for the generation of iPSCs; examples include Applied StemCell, Applied Biological Materials, Creative Bioarray, Lonza and Stemnovate.
- Given the various advantages associated with fibroblasts, such as easy availability, faster proliferation rate and cell robustness, these cells have found maximum (>40%) usage in cell reprogramming.
- Featuring the presence of several small and mid-sized firms, the cell reprogramming landscape is well distributed across various regions; players have established multiple facilities to cater to the needs of their clients.
- North America is a key services hub for cell reprogramming. More than 55% of companies engaged in cell reprogramming are headquartered in North America. This is followed by players based in Europe (45%).

- Prominent facilities within Europe include the UK, Germany, Denmark, Switzerland, France, Portugal and Sweden. Stakeholders are actively expanding their capabilities in order to enhance their respective cell reprogramming service portfolios and, thereby, maintain a competitive edge in this upcoming industry.
- The rising interest in this field is reflected in the number of partnerships, involving a varied range of cell types, inked in the recent past; the maximum partnering activity has been observed in the US.
- Since 2016, the partnership activity has increased at a CAGR of 7%; with the increasing demand for stem cells, both industry and academic players are likely to enter into multiple alliances for cell reprogramming.
- Both established players and new entrants have forged several strategic partnerships in the recent past; majority (>30%) of the deals have primarily been inked for generations of iPSCs, which are further differentiated based on the client's requirement.
- A number of companies have signed multiple deals for cell reprogramming; examples of the firms that have signed over four deals include Cellaria, BlueRock Therapeutics, FUJIFILM Cellular Dynamics and STEMCELL Technologies.
- In the last few years, over 540 clinical trials, evaluating various types of stem cell therapies have been initiated; this highlights the rising demand for iPSC generation and direct reprogramming strategies.
- >70% of these trials were / are being sponsored by small / mid-sized companies to exploit the potential of stem cell therapies to cater to the unmet need in this domain.
- An evaluation of more than 300+ stakeholders engaged in the development of stem cell therapies reveals several likely strategic partners for cell reprogramming service providers, across different geographical regions.
- The market is anticipated to grow at a CAGR of 8% till 2030, and the opportunity is likely to be distributed across different types of technologies, applications and key geographical regions.

- Presently, the majority share of service revenues is generated from projects involving episomal reprogramming technologies.
- In 2030, episomal reprogramming focused projects are anticipated to contribute over 40% of the overall share; it is expected to be followed by revenues generated from projects involving Sendai virus-based reprogramming (30%) and mRNA (25%).
Example Players in the Cell Reprogramming Market
- Allele Biotechnology
- ALSTEM
- Applied Biological Materials
- Axol Bioscience
- Creative Bioarray
- DefiniGEN
- FUJIFILM Cellular Dynamics
- Lonza
- Mogrify
- REPROCELL
- Stemnovate
- Thermo Fisher Scientific
GLOBAL CELL REPROGRAMMING MARKET: RESEARCH COVERAGE
- Market Sizing and Opportunity Analysis: The report features an in-depth analysis of the global cell reprogramming market, focusing on key market segments, including [A] type of technology, [B] type of source cell, [C] type of application and [D] key geographical regions.
- Market Landscape: A comprehensive evaluation of cell reprogramming market, considering various parameters, such as [A] year of establishment, [B] company size, [C] geographical location, [D] location of manufacturing facilities, [E] type of service, [F] type of offering, [G] type of technology, [H] type of vector used, [I] source cell for iPSC generation, [J] target indication(s), [K] type of application and [L] additional service(s) offered.
- Company Competitiveness Analysis: A comprehensive company competitive analysis of iPSC generation and direct reprogramming service providers, examining factors, such as [A] supplier strength and [B] service strength.
- Company Profiles: In-depth profiles of the players that offer cell reprogramming services and products, focusing on [A] company overview, [B] financial information (if available), [C] details on cell reprogramming approaches, [D] types of cell(s) utilized and differentiated, [E] target indication(s), [F] other drug discovery services offered and [G] recent developments and an informed future outlook.
- Clinical Trial Analysis: An insightful analysis more than 540 completed, ongoing and planned clinical studies of various stem cell therapies, based on several parameters, such as [A] trial registration year, [B] phase of development, [C] study design, [D] current trial status, [E] leading industry sponsors, [F] study focus, [G] type of stem cells, [H] target indication(s), [I] target therapeutic area(s), [J] enrolled patient population and [K] regional distribution of trials.
- Partnerships and Collaborations: An insightful analysis of the deals inked by stakeholders in the cell reprogramming market, based on several parameters, such as [A] year of partnership, [B] type of partnership, [C] type of stem cell, [D] target therapeutic area, [E] application, [F] most active players (in terms of the number of partnerships signed) and [G] geography.
- Likely Partnership Opportunities: A detailed discussion on the stem cell therapy developers / manufacturers that are anticipated to partner with cell reprogramming service and product providers in the foreseen future, based on various relevant parameters, such as [A] company size, [B] type of cell (s), [C] indication counts and [D] existing partnership(s).
KEY QUESTIONS ANSWERED IN THIS REPORT
- How many companies are currently engaged in this market?
- Which are the leading companies in this market?
- What factors are likely to influence the evolution of this market?
- What is the current and future market size?
- What is the CAGR of this market?
- How is the current and future market opportunity likely to be distributed across key market segments?
REASONS TO BUY THIS REPORT
- The report provides a comprehensive market analysis, offering detailed revenue projections of the overall market and its specific sub-segments. This information is valuable to both established market leaders and emerging entrants.
- Stakeholders can leverage the report to gain a deeper understanding of the competitive dynamics within the market. By analyzing the competitive landscape, businesses can make informed decisions to optimize their market positioning and develop effective go-to-market strategies.
- The report offers stakeholders a comprehensive overview of the market, including key drivers, barriers, opportunities, and challenges. This information empowers stakeholders to stay abreast of market trends and make data-driven decisions to capitalize on growth prospects.
ADDITIONAL BENEFITS
- Complimentary PPT Insights Packs
- Complimentary Excel Data Packs for all Analytical Modules in the Report
- 15% Free Content Customization
- Detailed Report Walkthrough Session with Research Team
- Free Updated report if the report is 6-12 months old or older
TABLE OF CONTENTS
1. PREFACE
- 1.1. Scope of the Report
- 1.2. Research Methodology
- 1.2.1. Research Assumptions
- 1.2.2. Project Methodology
- 1.2.3. Forecast Methodology
- 1.2.4. Robust Quality Control
- 1.2.5. Key Considerations
- 1.2.5.1. Demographics
- 1.2.5.2. Economic Factors
- 1.2.5.3. Government Regulations
- 1.2.5.4. Supply Chain
- 1.2.5.5. COVID Impact / Related Factors
- 1.2.5.6. Market Access
- 1.2.5.7. Healthcare Policies
- 1.2.5.8. Industry Consolidation
- 1.3 Key Questions Answered
- 1.4. Chapter Outlines
2. EXECUTIVE SUMMARY
3. INTRODUCTION
- 3.1. Chapter Overview
- 3.2. Overview of Stem Cells
- 3.2.1. Classification of Stem Cells
- 3.2.1.1. Based on Source of Stem Cell
- 3.2.1.2. Based on Origin of Stem Cell
- 3.2.1.3. Based on Potency of Stem Cell
- 3.2.2. Routes of Administration of Stem Cell Therapies
- 3.2.3. Applications of Stem Cell Therapies
- 3.2.1. Classification of Stem Cells
- 3.3. Introduction to Cell Reprogramming
- 3.3.1. Cell Reprogramming Approaches and Affiliated Technologies
- 3.4. Applications of Cell Reprogramming
- 3.5. Key Growth Drivers and Constraints
4. CURRENT MARKET LANDSCAPE
- 4.1. Chapter Overview
- 4.2. Cell Reprogramming Service and Product Providers: List of Industry Players
- 4.2.1. Analysis by Year of Establishment
- 4.2.2. Analysis by Company Size
- 4.2.3. Analysis by Geographical Location
- 4.2.4. Analysis by Location of Stem Cell Research Facilities
- 4.2.5. Analysis by Type of Service
- 4.2.6. Analysis by Type of Technology
- 4.2.7. Analysis by Target Indication
- 4.2.8. Analysis by Source Cell
- 4.2.9. Analysis by Application Area
- 4.2.10. Analysis by Additional Service(s) Offered
- 4.2.11. Analysis by Type of Offering
- 4.3. Cell Reprogramming Service and Product Providers: List of Non-Industry Players
- 4.3.1. Analysis by Geographical Location
- 4.3.2. Analysis by Type of Technology Used
- 4.3.3. Analysis by Source Cell for iPSC Generation
- 4.3.4. Analysis by Type of Offering
- 4.4. Cell Reprogramming: List of Affiliated Products Providers
5. COMPANY COMPETITIVENESS ANALYSIS
- 5.1. Chapter Overview
- 5.2. Assumptions and Key Parameters
- 5.3. Methodology
- 5.4. Competitiveness Analysis: iPSC Generation Service and Product Providers
- 5.5. Competitiveness Analysis: Direct Reprogramming Service and Product Providers
6. COMPANY PROFILES
- 6.1. Chapter Overview
- 6.2. Allele Biotechnology
- 6.2.1. Company Overview
- 6.2.2. Recent Developments and Future Outlook
- 6.3. ALSTEM
- 6.3.1. Company Overview
- 6.3.2. Recent Developments and Future Outlook
- 6.4. Applied Biological Materials
- 6.4.1. Company Overview
- 6.4.2. Recent Developments and Future Outlook
- 6.5. Axol Bioscience
- 6.5.1. Company Overview
- 6.5.2. Recent Developments and Future Outlook
- 6.6. Creative Bioarray
- 6.6.1. Company Overview
- 6.6.1.1. Recent Developments and Future Outlook
- 6.6.1. Company Overview
- 6.7. DefiniGEN
- 6.7.1. Company Overview
- 6.7.2. Recent Developments and Future Outlook
- 6.8. FUJIFILM Cellular Dynamics International
- 6.8.1. Company Overview
- 6.8.2. Recent Developments and Future Outlook
- 6.9. Lonza
- 6.9.1. Company Overview
- 6.9.2. Recent Developments and Future Outlook
- 6.10. Mogrify
- 6.10.1. Company Overview
- 6.10.2. Recent Developments and Future Outlook
- 6.11. REPROCELL
- 6.11.1. Company Overview
- 6.11.1.1. Recent Developments and Future Outlook
- 6.11.1. Company Overview
- 6.12. Stemnovate
- 6.12.1. Company Overview
- 6.12.2. Recent Developments and Future Outlook
- 6.14. Thermo fisher Scientific
- 6.14.1. Company Overview
- 6.14.1.1. Recent Developments and Future Outlook
- 6.14.1. Company Overview
7. CASE STUDY: CLINICAL TRIAL ACTIVITY IN STEM CELL THERAPY DEVELOPMENT
- 7.1. Chapter Overview
- 7.2. Scope and Methodology
- 7.3. Stem Cell Therapies: Clinical Trial Analysis
- 7.3.1. Analysis by Trial Registration Year
- 7.3.2. Analysis by Trial Phase
- 7.3.3. Analysis by Number of Patients Enrolled by Trial Registration Year
- 7.3.4. Analysis by Study Design
- 7.3.5. Analysis by Trial Recruitment Status
- 7.3.6. Analysis by Sponsor / Collaborator
- 7.3.7. Leading Industry Sponsors: Analysis by Number of Registered Trials
- 7.3.8. Analysis by Trial Focus
- 7.3.9. Analysis by Type of Stem Cell
- 7.3.10. Analysis by Therapeutic Area
- 7.3.11. Analysis by Type of Stem Cell and Therapeutic Area
- 7.3.12. Geographical Analysis by Number of Clinical Trials
- 7.3.13. Geographical Analysis by Trial Recruitment Status
- 7.3.14. Geographical Analysis by Enrolled Patient Population
8. PARTNERSHIPS AND COLLABORATIONS
- 8.1. Chapter Overview
- 8.2. Scope and Methodology
- 8.3. Cell Reprogramming Services and Products Market: List of Partnerships and Collaborations
- 8.3.1. Analysis by Year of Partnership
- 8.3.2. Analysis by Type of Partnership
- 8.3.3. Analysis by Year of Partnership and Type of Partner
- 8.3.4. Analysis by Type of Partnership and Type of Partner
- 8.3.5. Analysis by Type of Stem Cell
- 8.3.6. Analysis by Target Therapeutic Area
- 8.3.7. Analysis by Application
- 8.3.8. Most Active Players: Analysis by Number of Partnerships
- 8.3.9. Regional Analysis
- 8.3.10. Intercontinental and Intracontinental Agreements
9. LIKELY PARTNERSHIP OPPORTUNITIES
- 9.1. Chapter Overview
- 9.2. Scope and Methodology
- 9.3. Scoring Criteria and Key Assumptions
- 9.4. Stem Cell Therapy Developers: Likely Partners for Cell Reprogramming Service and Product Providers
- 9.4.1. Likely Partners for Cell Reprogramming Service and Product Providers in North America
- 9.4.2. Likely Partners for Cell Reprogramming Service and Product Providers in Europe
- 9.4.3. Likely Partners for Cell Reprogramming Service and Product Providers in Asia-Pacific and Rest of the World
- 9.5. Stem Cell Therapy Contract Manufacturers: Likely Partners for Cell Reprogramming Service and Product Providers
- 9.5.1. Likely Partners for Cell Reprogramming Service and Product Providers in North America
- 9.5.2. Likely Partners for Cell Reprogramming Service and Product Providers in Europe
- 9.5.3. Likely Partners for Cell Reprogramming Service and Product Providers in Asia-Pacific and Rest of the World
10. MARKET FORECAST
- 10.1. Chapter Overview
- 10.2. Scope and Methodology
- 10.3. Forecast Methodology and Key Assumptions
- 10.4. Global Cell Reprogramming Services and Products Market, Till 2035
- 10.4.1. Global Cell Reprogramming Services and Products Market: Distribution by Type of Technology, Till 2035
- 10.4.1.1. Cell Reprogramming Services and Products Market for Episomal Reprogramming, Till 2035
- 10.4.1.2. Cell Reprogramming Services and Products Market for mRNA Reprogramming, Till 2035
- 10.4.1.3. Cell Reprogramming Services and Products Market for Sendai Virus-based Reprogramming, Till 2035
- 10.4.1.4. Cell Reprogramming Services and Products Market for Other Reprogramming Technologies, Till 2035
- 10.4.2. Global Cell Reprogramming Services and Products Market: Distribution by Type of Source Cell, Till 2035
- 10.4.2.1. Cell Reprogramming Services and Products Market for Fibroblasts, Till 2035
- 10.4.2.2. Cell Reprogramming Services and Products Market for Peripheral Blood Mononuclear Cells, Till 2035
- 10.4.2.3. Cell Reprogramming Services and Products Market for Unspecified Somatic Cells, Till 2035
- 10.4.2.4. Cell Reprogramming Services and Products Market for Other Cells, Till 2035
- 10.4.3. Global Cell Reprogramming Services and Products Market: Distribution by Type of Application, Till 2035
- 10.4.3.1. Cell Reprogramming Services and Products Market for Research, Till 2035
- 10.4.3.2. Cell Reprogramming Services and Products Market for Therapeutic, Till 2035
- 10.4.4. Global Cell Reprogramming Services and Products Market: Distribution by Key Geographical Regions, Till 2035
- 10.4.4.1. Cell Reprogramming Services and Products Market in North America, Till 2035
- 10.4.4.2. Cell Reprogramming Services and Products Market in Europe, Till 2035
- 10.4.4.3. Cell Reprogramming Services and Products Market in Asia-Pacific and Rest of the World, Till 2035
- 10.4.1. Global Cell Reprogramming Services and Products Market: Distribution by Type of Technology, Till 2035
11. EXECUTIVE INSIGHTS
- 11.1. Chapter Overview
- 11.2. Company A
- 11.2.1. Company Snapshot
- 11.2.2. Interview Transcript: Co-Founder and Chief Executive Officer
- 11.3. Company B
- 11.3.1. Company Snapshot
- 11.3.2. Interview Transcript: Chief Scientific Officer
12. CONCLUDING REMARKS
13. APPENDIX 1: TABULATED DATA
14. APPENDIX 2: LIST OF COMPANIES AND ORGANIZATIONS
List of Tables
- Table 3.1 Differences between Stem Cell Therapies and Other Biologics
- Table 4.1 Cell Reprogramming Services and Products: List of Industry Players
- Table 4.2 Cell Reprogramming Services and Products: List of Non-Industry Players
- Table 4.3 Cell Reprogramming Services and Products: List of Affiliated Product Providers
- Table 6.1 Cell Reprogramming Service and Product Providers: List of Profiled Companies
- Table 6.2 ALSTEM: Company Snapshot
- Table 6.3 ALSTEM: Recent Developments and Future Outlook
- Table 6.4 Applied Biological Materials: Company Snapshot
- Table 6.5 Applied Biological Materials: Recent Developments and Future Outlook
- Table 6.6 Axol Bioscience: Company Snapshot
- Table 6.7 Axol Bioscience: Recent Developments and Future Outlook
- Table 6.8 Creative Bioarray: Company Snapshot
- Table 6.9 Creative Bioarray: Recent Developments and Future Outlook
- Table 6.10 DefiniGEN: Company Snapshot
- Table 6.11 DefiniGEN: Recent Developments and Future Outlook
- Table 6.12 FUJIFILM Cellular Dynamics International: Company Snapshot
- Table 6.13 FUJIFILM Cellular Dynamics International: Recent Developments and Future Outlook
- Table 6.14 Lonza: Company Snapshot
- Table 6.15 Lonza: Recent Developments and Future Outlook
- Table 6.16 Mogrify: Company Snapshot
- Table 6.17 Mogrify: Recent Developments and Future Outlook
- Table 6.18 REPROCELL: Company Snapshot
- Table 6.19 REPROCELL: Recent Developments and Future Outlook
- Table 6.20 Stemnovate: Company Snapshot
- Table 6.21 Stemnovate: Recent Developments and Future Outlook
- Table 6.22 Thermo Fisher Scientific: Company Snapshot
- Table 6.23 Thermo Fisher Scientific: Recent Developments and Future Outlook
- Table 7.1 Cell Reprogramming Services: List of Partnerships and Collaborations
- Table 9.1 Likely Partners for Cell Reprogramming Service and Product Providers in North America
- Table 9.2 Likely Partners for Cell Reprogramming Service and Product Providers in Europe
- Table 9.3 Likely Partners for Cell Reprogramming Service and Product Providers in Asia-Pacific and Rest of the World
- Table 9.4 Likely Partners for Cell Reprogramming Service and Product Providers in North America
- Table 9.5 Likely Partners for Cell Reprogramming Service and Product Providers in Europe
- Table 9.6 Likely Partners for Cell Reprogramming Service and Product Providers in Asia-Pacific and Rest of the World
- Table 11.1 Asgard Therapeutics: Company Snapshot
- Table 11.2 Phenocell: Company Snapshot
- Table 13.1 Cell Reprogramming Service and Product Providers (Industry Players): Distribution by Year of Establishment
- Table 13.2 Cell Reprogramming Service and Product Providers (Industry Players): Distribution by Company Size
- Table 13.3 Cell Reprogramming Service and Product Providers (Industry Players): Distribution by Geographical Location
- Table 13.4 Cell Reprogramming Service and Product Providers (Industry Players): Distribution by Location of Manufacturing Facilities
- Table 13.5 Cell Reprogramming Service and Product Providers (Industry Players): Distribution by Type of Service
- Table 13.6 Cell Reprogramming Service and Product Providers (Industry Players): Distribution by Type of Offering
- Table 13.7 Cell Reprogramming Service and Product Providers (Industry Players): Distribution by Type of Technology
- Table 13.8 Cell Reprogramming Service and Product Providers (Industry Players): Distribution by Type of Technology and Year of Establishment
- Table 13.9 Cell Reprogramming Service and Product Providers (Industry Players): Distribution by Type of Vector Used
- Table 13.10 Cell Reprogramming Service and Product Providers (Industry Players): Distribution by Source Cell for iPSC Generation
- Table 13.11 Cell Reprogramming Service and Product Providers (Industry Players): Distribution by Type of Source Cell for iPSC Generation and company size
- Table 13.12 Cell Reprogramming Service and Product Providers (Industry Players): Distribution by Target Indication
- Table 13.13 Cell Reprogramming Service and Product Providers (Industry Players): Distribution by Type of directly reprogrammed cell
- Table 13.14 Cell Reprogramming Service and Product Providers (Industry Players): Distribution by Type of Application
- Table 13.15 Cell Reprogramming Service and Product Providers (Industry Players): Distribution by Type of Additional Service(s) Offered
- Table 13.16 Cell Reprogramming Service and Product Providers (Non-Industry Players): Distribution by Geographical Location
- Table 13.17 Cell Reprogramming Service and Product Providers (Non-Industry Players): Distribution by Type of Technology
- Table 13.18 Cell Reprogramming Service and Product Providers (Non-Industry Players): Distribution by Type of Vector Used
- Table 13.19 Cell Reprogramming Service and Product Providers (Non-Industry Players): Distribution by Source Cell for iPSC Generation
- Table 13.20 Clinical Trial Analysis: Distribution by Trial Status
- Table 13.21 Clinical Trial Analysis: Cumulative Distribution of Trials by Registration Year, Since Pre-2010
- Table 13.22 Clinical Trial Analysis: Distribution by Trial Phase
- Table 13.23 Clinical Trial Analysis: Distribution by Number of Patients Enrolled by Trial Registration Year, Since 2011
- Table 13.24 Clinical Trial Analysis: Distribution by Study Design
- Table 13.25 Clinical Trial Analysis: Cumulative Year-wise Trend by Trial Recruitment Status
- Table 13.26 Clinical Trial Analysis: Distribution by Type of Sponsor / Collaborator
- Table 13.27 Clinical Trial Analysis: Leading Industry Players
- Table 13.28 Clinical Trial Analysis: Analysis by Type of Stem Cell
- Table 13.29 Clinical Trial Analysis: Cumulative Distribution by Type of Stem Cell and Trial Registration Year
- Table 13.30 Clinical Trial Analysis: Distribution by Therapeutic Area
- Table 13.31 Clinical Trial Analysis: Year-Wise Trend in Activity for Popular Therapeutic Areas
- Table 13.32 Clinical Trial Analysis: Distribution by Patient Enrollment and Therapeutic Area
- Table 13.33 Clinical Trial Analysis: Year-Wise Trend in Patient Enrollment (in thousands) for Popular Therapeutic Areas
- Table 13.34 Clinical Trial Analysis: Distribution by Type of Stem Cell and Therapeutic Area
- Table 13.35 Clinical Trial Analysis: Geographical Distribution by Number of Trials
- Table 13.36 Clinical Trial Analysis: Geographical Distribution by Trial Registration Year and Recruitment Status
- Table 13.37 Clinical Trial Analysis: Geographical Distribution by Enrolled Patient
- Table 13.38 Partnerships and Collaborations: Analysis by Year of Partnership
- Table 13.39 Partnerships and Collaborations: Analysis by Type of Partnership
- Table 13.40 Partnerships and Collaborations: Analysis by Year of Partnership and Type of Partner
- Table 13.41 Partnerships and Collaborations: Analysis by Type of Partnership and Type of Partner
- Table 13.42 Partnerships and Collaborations: Analysis by Type of Stem Cell
- Table 13.43 Partnerships and Collaborations: Analysis by Target Therapeutic Area
- Table 13.44 Partnerships and Collaborations: Analysis by Application
- Table 13.45 Most Active Players: Distribution by Number of Partnerships
- Table 13.46 Partnerships and Collaborations: Regional Analysis
- Table 13.47 Partnerships and Collaborations: Intercontinental and Intracontinental Agreements
- Table 13.48 Global Cell Reprogramming Services and Products Market, Till 2035 (USD Million)
- Table 13.49 Global Cell Reprogramming Services and Products Market: Distribution by Type of Technology, Till 2035
- Table 13.50 Cell Reprogramming Services and Products Market for Episomal Reprogramming, Till 2035 (USD Million)
- Table 13.51 Cell Reprogramming Services and Products Market for mRNA Reprogramming, Till 2035 (USD Million)
- Table 13.52 Cell Reprogramming Services and Products Market for Sendai Virus-based Reprogramming, Till 2035 (USD Million)
- Table 13.53 Cell Reprogramming Services and Products Market for Other Reprogramming Technologies, Till 2035 (USD Million)
- Table 13.54 Global Cell Reprogramming Services and Products Market: Distribution by Source Cell for iPSC Generation, Till 2035
- Table 13.55 Cell Reprogramming Services and Products Market for Fibroblasts, Till 2035 (USD Million)
- Table 13.56 Cell Reprogramming Services and Products Market for Peripheral Blood Mononuclear Cells, Till 2035 (USD Million)
- Table 13.57 Cell Reprogramming Services and Products Market for Unspecified Somatic Cells, Till 2035 (USD Million)
- Table 13.58 Cell Reprogramming Services and Products Market for Other Cells, Till 2035 (USD Million)
- Table 13.59 Global Cell Reprogramming Services and Products Market: Distribution by Application, Till 2035
- Table 13.60 Cell Reprogramming Services and Products Market for Research, Till 2035 (USD Million)
- Table 13.61 Cell Reprogramming Services and Products Market for Therapeutic Use, Till 2035 (USD Million)
- Table 13.62 Global Cell Reprogramming Services and Products Market: Distribution by Key Geographical Regions, Till 2035
- Table 13.63 Cell Reprogramming Services and Products Market in North America, Till 2035 (USD Million)
- Table 13.64 Cell Reprogramming Services and Products Market in Europe, Till 2035 (USD Million)
- Table 13.65 Cell Reprogramming Services and Products Market in Asia-Pacific and Rest of the World, Till 2035 (USD Million)
List of Figures
- Figure 3.1 Cellular Lineages used for Isolation of Stem Cells
- Figure 3.2 Steps Involved in the Development and Administration of Stem Cells
- Figure 3.3 Classification of Stem Cells
- Figure 3.4 Classification based on Source of Stem Cell
- Figure 3.5 Differences between Allogeneic and Autologous Stem Cell Therapies
- Figure 3.6 Classification of Stem Cells based on Origin
- Figure 3.7 Classification of Adult Stem Cells
- Figure 3.8 Classification based on Potency of Stem Cell
- Figure 3.9 Key Application Areas for Stem Cell Therapies
- Figure 3.10 Potential Applications of Cell Reprogramming
- Figure 3.11 Key Growth Drivers and Constraints to Cell Reprogramming
- Figure 4.1 Cell Reprogramming Service and Product Providers (Industry Players): Distribution by Year of Establishment
- Figure 4.2 Cell Reprogramming Service and Product Providers (Industry Players): Distribution by Company Size
- Figure 4.3 Cell Reprogramming Service and Product Providers (Industry Players): Distribution by Geographical Location
- Figure 4.4 Cell Reprogramming Service and Product Providers (Industry Players): Distribution by Location of Manufacturing Facilities
- Figure 4.5 Cell Reprogramming Service and Product Providers (Industry Players): Distribution by Type of Service
- Figure 4.6 Cell Reprogramming Service and Product Providers (Industry Players): Distribution by Type of Offering
- Figure 4.7 Cell Reprogramming Service and Product Providers (Industry Players): Distribution by Type of Technology
- Figure 4.8 Cell Reprogramming Service and Product Providers (Industry Players): Distribution by Type of Technology and Year of Establishment
- Figure 4.9 Cell Reprogramming Service and Product Providers (Industry Players): Distribution by Type of Vector Used
- Figure 4.10 Cell Reprogramming Service and Product Providers (Industry Players): Distribution by Source Cell for iPSC Generation
- Figure 4.11 Cell Reprogramming Service and Product Providers (Industry Players): Distribution by Source Cell for iPSC Generation and Company size
- Figure 4.12 Cell Reprogramming Service and Product Providers (Industry Players): Distribution by Target Indication
- Figure 4.13 Cell Reprogramming Service and Product Providers (Industry Players): Distribution by Type of Directly Reprogrammed cells
- Figure 4.14 Cell Reprogramming Service and Product Providers (Industry Players): Distribution by Type of Application
- Figure 4.15 Cell Reprogramming Service and Product Providers (Industry Players): Distribution by Type of Additional Service(s) Offered
- Figure 4.16 Cell Reprogramming Service and Product Providers (Non-Industry Players): Distribution by Geographical Location
- Figure 4.17 Cell Reprogramming Service and Product Providers (Non-Industry Players): Distribution by Type of Technology
- Figure 4.18 Cell Reprogramming Service and Product Providers (Non-Industry Players): Distribution by Type of Vector Used
- Figure 4.19 Cell Reprogramming Service and Product Providers (Non-Industry Players): Distribution by Source Cell for iPSC Generation
- Figure 5.1 Competitiveness Analysis of iPSCs Generation Service and Product Providers
- Figure 5.2 Competitiveness Analysis of Direct Reprogramming Service and Product Providers
- Figure 7.1 Clinical Trial Analysis: Scope and Methodology
- Figure 7.2 Clinical Trial Analysis: Distribution by Trial Status
- Figure 7.3 Clinical Trial Analysis: Cumulative Distribution of Trials by Registration Year, Since Pre-2010
- Figure 7.4 Clinical Trial Analysis: Distribution by Trial Phase
- Figure 7.5 Clinical Trial Analysis: Distribution by Number of Patients Enrolled and Trial Registration Year, Since 2011
- Figure 7.6 Clinical Trial Analysis: Distribution by Study Design
- Figure 7.7 Clinical Trial Analysis: Cumulative Year-wise Trend by Trial Recruitment Status
- Figure 7.8 Clinical Trial Analysis: Distribution by Type of Sponsor / Collaborator
- Figure 7.9 Clinical Trial Analysis: Leading Industry Players
- Figure 7.10 Clinical Trial Analysis: Distribution by Focus Area
- Figure 7.11 Clinical Trial Analysis: Distribution by Type of Stem Cell
- Figure 7.12 Clinical Trial Analysis: Cumulative Distribution by Type of Stem Cell and Trial Registration Year
- Figure 7.13 Clinical Trial Analysis: Distribution by Therapeutic Area
- Figure 7.14 Clinical Trial Analysis: Year-Wise Trend in Activity for Popular Therapeutic Areas
- Figure 7.15 Clinical Trial Analysis: Distribution by Patient Enrollment and Therapeutic Area
- Figure 7.16 Clinical Trial Analysis: Year-Wise Trend in Patient Enrollment (in thousands) for Popular Therapeutic Areas
- Figure 7.17 Clinical Trial Analysis: Distribution by Type of Stem Cell and Therapeutic Area
- Figure 7.18 Clinical Trial Analysis: Geographical Distribution by Number of Trials
- Figure 7.19 Clinical Trial Analysis: Geographical Distribution by Trial Registration Year and Recruitment Status
- Figure 7.20 Clinical Trial Analysis: Geographical Distribution by Enrolled Patient Population
- Figure 8.1 Partnerships and Collaborations: Distribution by Year of Partnership
- Figure 8.2 Partnerships and Collaborations: Distribution by Type of Partnership
- Figure 8.3 Partnerships and Collaborations: Distribution by Year of Partnership and Type of Partner
- Figure 8.4 Partnerships and Collaborations: Distribution by Type of Partnership and Type of Partner
- Figure 8.5 Partnerships and Collaborations: Distribution by Type of Stem Cell
- Figure 8.6 Partnerships and Collaborations: Distribution by Target Therapeutic Area
- Figure 8.7 Partnerships and Collaborations: Distribution by Application
- Figure 8.8 Most Active Players: Distribution by Number of Partnerships
- Figure 8.9 Partnerships and Collaborations: Regional Analysis
- Figure 8.10 Partnerships and Collaborations: Intercontinental and Intracontinental Agreement
- Figure 10.1 Global Cell Reprogramming Services and Products Market, Till 2035 (USD Million)
- Figure 10.2 Global Cell Reprogramming Services and Products Market: Distribution by Type of Technology, Till 2035
- Figure 10.3 Cell Reprogramming Services and Products Market for Episomal Reprogramming, Till 2035 (USD Million)
- Figure 10.4 Cell Reprogramming Services and Products Market for mRNA Reprogramming, Till 2035 (USD Million)
- Figure 10.5 Cell Reprogramming Services and Products Market for Sendai Virus-based Reprogramming, Till 2035 (USD Million)
- Figure 10.6 Cell Reprogramming Services and Products Market for Other Reprogramming Technologies, Till 2035 (USD Million)
- Figure 10.7 Global Cell Reprogramming Services and Products Market: Distribution by Source Cell for iPSC Generation, Till 2035
- Figure 10.8 Cell Reprogramming Services and Products Market for Fibroblasts, Till 2035 (USD Million)
- Figure 10.9 Cell Reprogramming Services and Products Market for Peripheral Blood Mononuclear Cells, Till 2035 (USD Million)
- Figure 10.10 Cell Reprogramming Services and Products Market for Unspecified Somatic Cells, Till 2035 (USD Million)
- Figure 10.11 Cell Reprogramming Services and Products Market for Other Cells, Till 2035 (USD Million)
- Figure 10.12 Global Cell Reprogramming Services and Products Market: Distribution by Application, Till 2035
- Figure 10.13 Cell Reprogramming Services and Products Market for Research, Till 2035 (USD Million)
- Figure 10.14 Cell Reprogramming Services and Products Market for Therapeutic Use, Till 2035 (USD Million)
- Figure 10.15 Global Cell Reprogramming Services and Products Market: Distribution by Key Geographical Regions, Till 2035
- Figure 10.16 Cell Reprogramming Services and Products Market in North America, Till 2035 (USD Million)
- Figure 10.17 Cell Reprogramming Services and Products Market in Europe, Till 2035 (USD Million)
- Figure 10.18 Cell Reprogramming Services and Products Market in Asia-Pacific and Rest of the World, Till 2035 (USD Million)







