 |
市场调查报告书
商品编码
1891247
临床试验中心支持机构市场:产业趋势及全球预测(至 2035 年)-按治疗领域、试验阶段、临床试验组成部分、介入类型和主要地区划分Site Management Organization Market: Industry Trends and Global Forecasts, Till 2035 - Distribution by Therapeutic Area, Trial Phases, Clinical Trial Components, Type of Interventions and Key Geographies |
||||||
临床试验中心支持机构市场:市场概览
据估计,临床试验中心支援机构市场规模将从今年的 124 亿美元增长至 2035 年的 316 亿美元,预测期内(至 2035 年)的复合年增长率 (CAGR) 为 9.8%。
临床试验中心支持机构市场:成长与趋势
临床试验是整个药物研发过程中至关重要的阶段,在评估候选药物的安全性和有效性方面发挥着不可或缺的作用。研究表明,用于候选药物研发的总投资中约有 40% 用于临床试验,每年的成本高达 780 亿美元。 然而,进行这些试验往往面临诸多挑战,包括科学和操作上的复杂性、招募和留住合适患者的挑战、数据管理方面的挑战以及严格的监管标准。
此外,整个流程固有的复杂性以及多方利害关係人的参与,使得这些试验容易延误。超过 80% 的临床试验会延误 1 至 6 个月,而只有 10% 的试验能够准时完成。因此,製药创新者不断努力改善临床试验的进行和有效的管理方法。在众多选择中,将各种试验职能外包给诸如临床试验中心管理机构 (SMO) 等专业服务提供者已成为许多研发者的热门选择。由于註册临床试验的数量和复杂性不断增加,预计在整个预测期内,对 SMO 的需求将稳定成长。
市场成长驱动因素:
临床试验中心管理机构 (SMO) 市场的成长动力源自于临床试验数量和复杂性的不断增加。这是因为 II/III 期试验和精准医疗的入组研究需要专业的现场支援。 此外,慢性病和罕见病患疾病率的不断上升,推动了对利用电子健康记录 (EHR) 和区域网路进行病患招募和留存的临床试验管理组织 (SMO) 专业知识的需求。向分散式和混合式试验的转变,推动了 SMO 在远端监查、数位资源和数据准确性方面的应用。此外,外包趋势、亚太和拉丁美洲新兴市场的成长,以及人工智慧和电子知情同意书 (eConsent) 等技术的整合,正在进一步推动 SMO 的发展。对真实世界数据 (RWE) 和上市后监测日益增长的需求,正将 SMO 转变为可扩展的高附加价值网路。
市场限制因素:
临床试验管理组织 (SMO) 市场面临着许多限制因素,包括员工培训、法规遵循和技术整合等高昂的营运成本,这使得 SMO 难以製定具有竞争力的价格。来自内部临床试验网络、旗下拥有 SMO 的大型合约研究组织 (CRO) 以及综合研究组织 (IRO) 的激烈竞争,正在阻碍 SMO 的市场占有率增长,迫使规模较小的 SMO 专注于特定领域。 病患招募和留存仍然是一大挑战,儘管临床试验支援组织 (SMO) 已尽力,但由于入组人数不足,导致试验严重延误。此外,对合约研究组织 (CRO) 与申办者之间合约的依赖以及新兴市场的经济波动,使得 SMO 的收入难以预测,阻碍了市场扩张。
临床试验支持组织 (SMO) 市场:主要发现
本报告深入分析了目前临床试验支持组织市场的现状,并指出了该行业的潜在成长机会。主要发现包括:
1. 目前,全球约有 250 家业者声称可为治疗产品和医疗器材的临床试验申办方提供临床试验支援服务。
2. 市场较为分散,既有老牌企业,也有新进入者,它们都声称能够从地理位置分散的地点,为各种治疗领域提供临床试验中心管理服务。
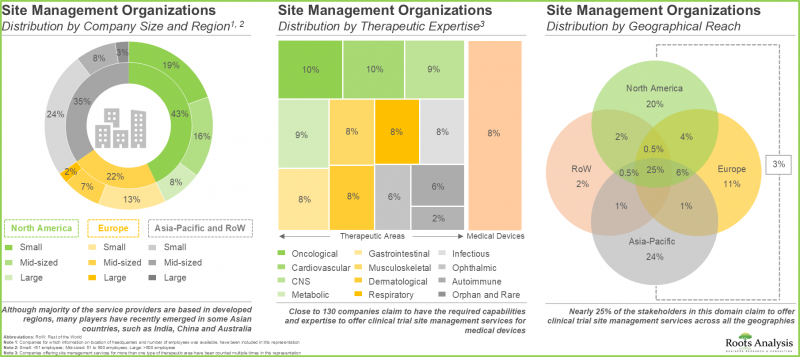
3. 该领域的公司正在稳步提升自身能力,以拓展服务组合併适应不断变化的行业标准。
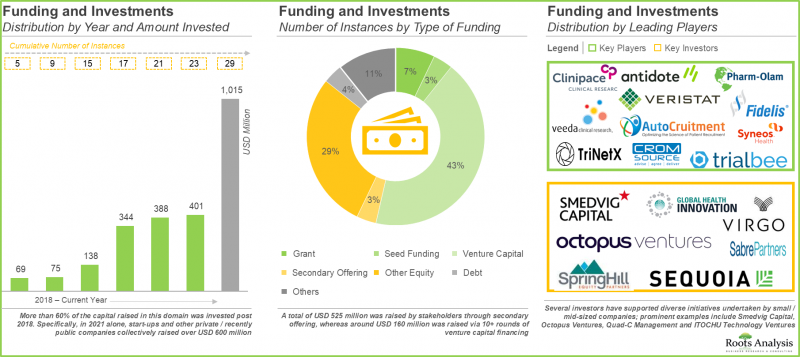
4. 在过去五年中,已签署超过200项协议,其中大部分合作集中在服务合作和临床试验协议方面,旨在为申办方公司提供临床研究服务。
5. 众多投资人看好未来的获利前景,已向29家临床试验中心支持服务公司投资总计10亿美元。
6. 2020年,全球註册的临床试验约有10,000项,旨在评估各种潜在干预措施,并开发循证医学和医疗保健解决方案。
7. 随着临床试验数量的持续增长,对研究参与者的需求也急剧上升,促使药物研发公司转向第三方服务提供者。
8. 目前超过60%的临床试验营运外包,预计未来十年该市场将保持稳定成长。
9. 预计市场机会可能分布在各个治疗领域和主要地理区域。
临床试验中心管理组织 (SMO) 市场
市场规模与机会分析依下列参数细分:
治疗领域
- 肿瘤学
- 中枢神经系统疾病
- 传染病
- 心血管疾病
- 其他
试验阶段
- I期
- II期
- III期
- IV期
临床试验组成
- 现场管理
- 现场监查
- 专案管理
- 其他
介入类型
- 治疗方法
- 医疗器材
- 外科手术
主要地区
- 北美
- 欧洲
- 世界其他地区
临床试验中心管理机构 (SMO) 市场:主要细分市场
预计 SMO 市场将主要由旨在治疗和管理肿瘤适应症的临床试验推动
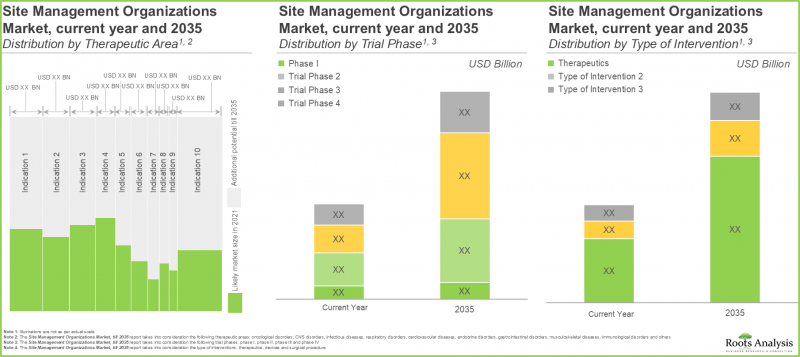
依治疗领域划分,SMO 市场可细分为肿瘤、中枢神经系统、传染病、呼吸系统、心血管系统、内分泌系统、胃肠道、肌肉骨骼系统、免疫系统、皮肤及其他。预计到 2035 年,SMO 市场将占 38% 的市场占有率,其中旨在治疗和管理肿瘤适应症的临床试验将发挥主导作用。这主要是由于目前全球范围内正在进行或计划进行的临床试验数量不断增加,这些试验旨在研究针对肿瘤适应症的药物的潜力。
预计在预测期内,II期临床试验的SMO市场将保持领先地位。
依临床试验阶段划分,整体市场分为I期、II期、III期及IV期。今年II期临床试验预计的SMO市场将占市场占有率的约40%,并在预测期内以12.1%的复合年增长率成长。
预计在预测期内,临床试验现场管理活动将引领临床试验现场支持组织市场。
就临床试验营运而言,整体市场分为现场管理、资料管理、品质控制、本地生产、法规事务、专案管理、物流和其他。预计今年现场管理活动(包括试验中心选择、合约签订和付款、试验中心启动和激活以及试验中心关闭)的SMO市场将占市场占有率的约30%,并在预测期内以9.8%的复合年增长率增长。
预计在预测期内,治疗药物(药品和生物製品)市场将以 9.8% 的复合年增长率成长。
就治疗药物而言,整体市场细分为外科手术、医疗器材和治疗药物。预计到 2035 年,治疗药物(药品和生物製品)SMO 市场规模将达到 220 亿美元,在预测期内以 9.8% 的复合年增长率成长。
预计北美将推动临床试验现场支持组织市场的成长。
按地区划分,整体市场细分为北美、欧洲、亚太地区、拉丁美洲、中东和北非 (MENA) 以及世界其他地区 (RoW)。
今年,北美占了整体临床试验现场支援市场的大部分占有率,预计这一趋势在可预见的未来将保持不变。 然而,亚太地区预计将呈现更快的成长,在预测期内复合年增长率将达到 13.7%。
临床试验现场支援机构市场最新动态:
临床试验现场支援机构领域近期出现了一些新的发展动态。以下列举了一些近期措施。虽然这些发展动态发生在我们的市场报告发布之后,但它们与我们分析中概述的整体市场趋势相符。
- Psyence Biomed 与澳洲临床试验网络 (ACTioN) 签署了策略合作协议,共同进行 IIb 期临床试验。
- Flourish Research 获得了 Genstar Capital 的策略性投资,以进一步拓展其临床研究服务。
- Neutra Corporation 收购了总部位于美国的临床试验现场支援机构 Mercury Clinical Research。此外,资产管理公司 Blackstone 宣布计划收购总部位于东京的临床试验现场支援机构 I'rom Group。
临床试验机构支持组织 (CRO) 市场代表性公司
- FOMAT Medical Research
- Parexel
- Pharm-Olam
- Veristat
- WCCT Global
- Worldwide Clinical Trials
- CROMSOURCE
- Fidelis Research
- Scandinavian CRO
- TFS Health Science
- Trialbee
- CMIC Group
- George Clinical
- Tigermed
- Veeda Clinical Research
目录
第一章:前言
第二章:摘要整理
第三章:导论
- 章节概要
- 临床试验中心支持机构
- 临床试验中心支援机构在临床试验中的作用
- 临床试验中心支援机构提供的服务
- 一站式服务的优势
- 结论
第四章:竞争格局
- 章节概要
- 临床试验中心支援机构:市场格局
第五章:竞争分析
- 章节概要
- 假设和关键参数
- 研究方法
- 临床试验中心支援机构:竞争分析
第六章 公司简介:北美临床试验中心支持机构
- 章节概述
- FOMAT Medical Research
- Parexel
- Pharm-Olam
- Veristat
- WCCT Global
- Worldwide Clinical Trials
第七章:公司简介:欧洲临床试验中心支持机构
- 章节概述
- CROMSOURCE
- Fidelis Research
- Scandinavian CRO
- TFS健康科学
- Trialbee
第八章:公司简介:亚太地区临床试验中心支持机构
- 章节概述
- CMIC集团
- George Clinical
- Tigermed
- Veeda Clinical Research
第九章:合作关係
- 章节概述
- 合作模式
- 临床试验中心支持机构:合作关係
第十章:资金与投资分析
- 章节概述
- 资金类型
- 临床试验中心支援机构:资金与投资分析
第 11 章:临床试验分析的主要的洞察
- 章节概述
- 研究范围与方法
- 临床试验中心支援机构:临床试验的主要的洞察
第 12 章:临床试验参与者需求分析
- 章节概述
- 研究方法与关键假设
- 全球临床试验参与者需求:按入组患者族群分析
第 13 章:市场预测与机会分析
- 章节概述
- 预测方法与关键假设
- 2035 年全球临床试验中心支援机构市场
- 2021 年及以后依治疗领域划分的临床试验中心支持机构市场2035 年
- 依试验阶段划分的临床试验现场管理机构市场,2035 年
- 依临床试验组成部分划分的临床试验现场管理机构市场,2035 年
- 按干预类型划分的临床试验现场管理机构市场,2035 年
- 按地区划分的临床试验现场管理机构市场,2021 年和 2035 年
第 14 章:结论
第 15 章:高阶主管洞察
第 16 章,附录 1:表格资料
第 17 章,附录 2:公司与机构清单
SITE MANAGEMENT ORGANIZATION MARKET: OVERVIEW
As per Roots Analysis, the site management organization market is estimated to grow from USD 12.4 billion in the current year to USD 31. 6 billion by 2035, at a CAGR of 9.8% during the forecast period, till 2035.
SITE MANAGEMENT ORGANIZATIONS MARKET: GROWTH AND TRENDS
Clinical trials are a crucial step of the entire drug development process, allowing for the essential assessment of a drug candidate's safety and effectiveness. Research indicates that approximately 40% of the overall investment allocated for the advancement of drug candidates is spent on clinical trials, amounting to an annual cost of USD 78 billion. Nonetheless, conducting such trials frequently presents significant difficulties, including scientific and operational intricacies, challenges with recruitment and retention of appropriate patients, challenges associated with data management, and rigorous regulatory standards.
Additionally, due to the intrinsic complexity of the entire process and the participation of multiple stakeholders, these trials are susceptible to delays. Over 80% of clinical trials experience delays ranging from one to six months, whereas merely 10% of the studies finish on schedule. Consequently, innovators within the pharmaceutical sector are consistently working on enhancing methods for executing clinical trials and managing them effectively. Among other options, delegating different trial operations to a dedicated service provider, such as site management organizations (SMOs), has become a prominent choice for many developers. As the complexity increases and the number of registered clinical trials rises, the need for SMOs is expected to see consistent market growth throughout the forecast period.
Market Growth Drivers:
The site management organization (SMO) market is driven by increasing clinical trial volume and complexity, as registered studies require specialized site assistance for Phase II / III and precision medicine. Moreover, the increasing prevalence of chronic and rare diseases drives the need for SMOs' expertise in patient recruitment and retention via EHRs and community networks. The transition to decentralized and hybrid trials boosts SMO implementation for overseeing remote monitoring, digital resources, and data accuracy. Further, trends in outsourcing, growth of emerging markets in Asia-Pacific and LATAM, along with technology integration such as AI and eConsent propel further expansion. It is worth highlighting that the need for real-world evidence and post-marketing research is transforming SMOs into scalable, high-value networks.
Market Restraints:
The Site Management Organization (SMO) market faces considerable limitations due to high operational expenses, such as employee training, adherence to regulations, and technology integration which present competitive pricing challenges. Severe rivalry from internal site networks, major CROs with incorporated SMOs, and rising integrated research organizations (IROs) hampers market share and compels smaller SMOs to focus on niche sectors. Recruitment and retention of patients continue to pose challenges, leading to considerable trial delays from insufficient enrollment, even with SMO initiatives. Ultimately, reliance on CRO-sponsor agreements and economic fluctuations in emerging markets subject SMOs to revenue unpredictability, thus hindering their market expansion.
SITE MANAGEMENT ORGANIZATIONS MARKET: KEY INSIGHTS
The report delves into the current state of the site management organizations market and identifies potential growth opportunities within industry. Some key findings from the report include:
1. Presently, around 250 players across the globe claim to offer clinical trial site management services to trial sponsors, for both therapeutic products and medical devices.
2. The market is fragmented, featuring the presence of both established players and new entrants based in different geographies that claim to be capable of offering site management services, for wide range of therapeutic areas.

3. Companies involved in this domain are steadily expanding their capabilities in order to enhance their respective service portfolios and comply to evolving industry benchmarks.
4. Over 200 deals have been inked in the past five years; majority of the reported collaborations were focused on forming service alliances and clinical trial agreements to offer clinical research services to sponsors.
5. Foreseeing a lucrative future, a large number of investors have invested capital worth USD 1 billion, across 29 instances, in companies offering clinical trial site management services.

6. In 2020, around 10,000 trials were registered to evaluate various types of potential interventions and develop evidence-based medicine and health care solutions, worldwide.
7. There has been a surge in the demand for study participants owing to the continuous growth in number of clinical trials; this has prompted drug developers to leverage services to third party service providers.
8. With more than 60% of the clinical trial operations currently being outsourced, we expect the market to grow at a steady pace over the next decade.

9. The anticipated opportunity is expected to be segregated across a variety of therapeutic areas; it is also likely to be well-distributed across key geographical regions.
Site Management Organization Market
The market sizing and opportunity analysis has been segmented across the following parameters:
Therapeutic Areas
- Oncological Disorders
- CNS Disorders
- Infectious Diseases
- Cardiovascular Diseases
- Others
Trial Phases
- Phase I
- Phase II
- Phase III
- Phase IV
Clinical Trial Components
- Site Management
- Onsite monitoring
- Project Management
- Others
Type of Interventions
- Therapeutics
- Devices
- Surgical Procedures
Key Geographies
- North America
- Europe
- Rest of the World
SITE MANAGEMENT ORGANIZATIONS MARKET: KEY SEGMENTS
SMOs Market is Likely to be Dominated by Trial Studies Intended for the Treatment / Management of Oncological Indications
In terms of therapeutic area, the market is segmented across oncological disorders, CNS disorders, infectious diseases, respiratory disorders, cardiovascular diseases, endocrine disorders, gastrointestinal disorders, musculoskeletal diseases, immunological disorders, dermatological disorders and others. The SMOs market is likely to be dominated by trial studies intended for the treatment / management of oncological indications, capturing 38% of the market share by 2035. This is owing to an increase in number of trials currently being / anticipated to be conducted to investigate the potential of drugs targeting oncological indications, worldwide.
SMOs market for Phase II Trial Studies is Likely to Dominate during the Forecast Period
In terms of the trial phase, the overall market is segmented across Phase I, Phase II, Phase III and Phase IV. The SMOs market for phase II trial studies is likely to capture nearly 40% of the market share in the current year, growing at a CAGR of 12.1%, during the given time period
Site Management Activities is Anticipated to Lead the Site Management Organization Market during the Forecast Period
In terms of clinical trial operations, the overall market is segmented across site management, data management, quality control, onsite manufacturing, regulatory affairs, project management, logistics and others. The SMOs market for site management activities (including site identification and selection, site contracting and payments, site initiation and activation and site close-out) is likely to capture around 30% of the market share in the current year, growing at a CAGR of 9.8%, during the given forecast period.
Market For Therapeutics (Drugs and Biologics) is Likely to Grow at a CAGR of 9.8%, During the Given Time Period
In terms of therapeutics, the overall market is segmented across surgical procedures, devices and therapeutics. the SMOs market for therapeutics (drugs and biologics) is likely to be worth USD 22 billion in 2035, growing at a CAGR of 9.8%, during the given time period.
North America is Likely to Propel the Growth of the Site Management Organization Market
In terms of geographical regions, the overall market is segmented across North America, Europe, Asia-Pacific, Latin America, MENA and RoW.
In the current year, North America captures the majority share of the overall clinical trial site management market and this trend is unlikely to change in the foreseen future. However, Asia-Pacific is likely to grow at a faster growth rate, with a CAGR of 13.7% during the forecast period.
Recent Developments in Site Management Organization Market:
Several recent developments have taken place in the field of site management organization. We have outlined some of these recent initiatives below. These developments, even if they took place post the release of our market report, substantiate the overall market trends that have been outlined in our analysis.
- Psyence Biomed entered into a strategic agreement with Australian Clinical Trial Network (ACTioN) for its Phase IIb clinical trial.
- Flourish Research received strategic investment from Genstar Capital to further expand its clinical research services.
- Neutra Corporation acquired Mercury Clinical Research, a US based site management organization. Blackstone, an asset management company, also announced its plans to acquire Tokyo based SMO I'rom Group.
Primary Research Overview
The opinions and insights presented in the market report were also influenced by discussions held with senior stakeholders in the industry. The market research report features detailed transcripts of interviews held with the following individuals:
- Country Head - Clinical Operations, Mid-sized Company, India
- Medical Director and Operations Manager, Small Company, Argentina
- Project Manager, Small Company, India
Example Players in Site Management Organizations Market
- FOMAT Medical Research
- Parexel
- Pharm-Olam
- Veristat
- WCCT Global
- Worldwide Clinical Trials
- CROMSOURCE
- Fidelis Research
- Scandinavian CRO
- TFS HealthScience
- Trialbee
- CMIC Group
- George Clinical
- Tigermed
- Veeda Clinical Research
SITE MANAGEMENT ORGANIZATIONS MARKET: RESEARCH COVERAGE
- Market Sizing and Opportunity Analysis: The report features an in-depth analysis of the site management organizations market, focusing on key market segments, including [A] therapeutic area, [B] trial phases, [C] clinical trial components, [D] type of interventions, and [E] key geographical regions.
- Market Landscape: A detailed assessment of overall competitive landscape companies offering clinical trial management services to various organizations, including CROs, and pharmaceutical, biotechnology and medical devices companies based on several relevant parameters, such as [A] year of establishment, [B] company size, [C] location of headquarters, [D] type of service offered, and [E] therapeutic expertise of service providers.
- Company Competitiveness Analysis: A comprehensive competitive analysis of service providers segregated into three peer groups based on location of their headquarters (North America, Europe, and Asia-Pacific and RoW), examining factors, such as [A] supplier strength [B] product strength and [C] application areas.
- Company Profiles: In-depth profiles of prominent players that offer various SMO services focusing on [A] year of establishment, [B] location of headquarters, [C] product portfolio, [D] recent developments and [E] an informed future outlook.
- Partnerships and Collaborations: An analysis of the partnerships that have been inked by stakeholders engaged in site management organization market, based on various parameters, such as [A] year of partnership, [B] type of partnership, [C] focus area and [D] most active players.
- Funding and Investment Analysis: A detailed analysis of various investments received by players engaged in site management organization market based on several relevant parameters, such as [A] year of investment, [B] number of funding instances, [C] amount invested, [D] type of funding (grant, seed, venture capital, secondary offering, other equity, debt and others) and [E] type of investor, [F] most active players, [G] most active investors and [H] geographical distribution (in terms of number of funding instances and amount invested).
- Clinical Trial Analysis: An in-depth analysis of completed, ongoing and planned clinical studies based on several relevant parameters, such as [A] trial registration year, [B] number of enrolled patients, [C] trial status, [D] trial phase, [E] type of sponsor and [F] geographical distribution of number of trials and enrolled patient population.
- Demand Analysis: An analysis of the annual demand for clinical study participants, taking into account the target patient population in ongoing and planned clinical trials, sponsored by both industry and non-industry players.
KEY QUESTIONS ANSWERED IN THIS REPORT
- What is a site-specific management organization and how does it operate?
- How many companies are currently engaged in this market?
- Which are the leading companies in this market?
- Which region dominates the site management organizations market?
- What are the key trends observed in the site management organizations market?
- What factors are likely to influence the evolution of this market?
- What are the primary challenges faced by site management organizations?
- What is the current and future market size?
- What is the CAGR of this market?
- How is the current and future market opportunity likely to be distributed across key market segments?
REASONS TO BUY THIS REPORT
- The report provides a comprehensive market analysis, offering detailed revenue projections of the overall market and its specific sub-segments. This information is valuable to both established market leaders and emerging entrants.
- Stakeholders can leverage the report to gain a deeper understanding of the competitive dynamics within the market. By analyzing the competitive landscape, businesses can make informed decisions to optimize their market positioning and develop effective go-to-market strategies.
- The report offers stakeholders a comprehensive overview of the market, including key drivers, barriers, opportunities, and challenges. This information empowers stakeholders to stay abreast of market trends and make data-driven decisions to capitalize on growth prospects.
ADDITIONAL BENEFITS
- Complimentary PPT Insights Packs
- Complimentary Excel Data Packs for all Analytical Modules in the Report
- 15% Free Content Customization
- Detailed Report Walkthrough Session with Research Team
- Free Updated report if the report is 6-12 months old or older
TABLE OF CONTENTS
1. PREFACE
- 1.1. Scope of the Report
- 1.2. Research Methodology
- 1.3. Key Questions Answered
- 1.4. Chapter Outlines
2. EXECUTIVE SUMMARY
3. INTRODUCTION
- 3.1. Chapter Overview
- 3.2. Site Management Organizations
- 3.3. Role of Site Management Organizations in Clinical Trials
- 3.4. Services Offered by Site Management Organizations
- 3.5. Advantages of One-Stop-Shops
- 3.6. Concluding Remarks
4. COMPETITIVE LANDSCAPE
- 4.1. Chapter Overview
- 4.2. Site Management Organizations: Overall Market Landscape
- 4.2.1. Analysis by Year of Establishment
- 4.2.2. Analysis by Company Size
- 4.2.3. Analysis by Location of Headquarters
- 4.2.4. Analysis by Service(s) Offered
- 4.2.5. Analysis by Therapeutic Expertise
- 4.2.6. Analysis by Geographical Reach
5. COMPANY COMPETITIVENESS ANALYSIS
- 5.1. Chapter Overview
- 5.2. Assumptions and Key Parameters
- 5.3. Methodology
- 5.4. Site Management Organizations: Company Competitiveness Analysis
- 5.4.1. Competitiveness Analysis: Site Management Organizations in North America
- 5.4.1.1. Competitiveness Analysis: Small Companies in North America
- 5.4.1.2. Competitiveness Analysis: Mid-sized Companies in North America
- 5.4.1.3. Competitiveness Analysis: Large Companies in North America
- 5.4.2. Competitiveness Analysis: Site Management Organizations in Europe
- 5.4.2.1. Competitiveness Analysis: Small Companies in Europe
- 5.4.2.2. Competitiveness Analysis: Mid-sized Companies in Europe
- 5.4.2.3. Competitiveness Analysis: Large Companies in Europe
- 5.4.3. Competitiveness Analysis: Site Management Organizations in Asia-Pacific and Rest of the World
- 5.4.3.1. Competitiveness Analysis: Small Companies in Asia-Pacific and Rest of the World
- 5.4.3.2. Competitiveness Analysis: Mid-sized Companies in Asia-Pacific and Rest of the World
- 5.4.3.3. Competitiveness Analysis: Large Companies in Asia-Pacific and Rest of the World
- 5.4.1. Competitiveness Analysis: Site Management Organizations in North America
6. COMPANY PROFILES: SITE MANAGEMENT ORGANIZATIONS IN NORTH AMERICA
- 6.1. Chapter Overview
- 6.2. FOMAT Medical Research
- 6.2.1. Company Overview
- 6.2.2. Clinical Trial Site Management Services
- 6.2.3. Recent Developments and Future Outlook
- 6.3. Parexel
- 6.3.1. Company Overview
- 6.3.2. Clinical Trial Site Management Services
- 6.3.3. Recent Developments and Future Outlook
- 6.4. Pharm-Olam
- 6.4.1. Company Overview
- 6.4.2. Clinical Trial Site Management Services
- 6.4.3. Recent Developments and Future Outlook
- 6.5. Veristat
- 6.5.1. Company Overview
- 6.5.2. Clinical Trial Site Management Services
- 6.5.3. Recent Developments and Future Outlook
- 6.6. WCCT Global
- 6.6.1. Company Overview
- 6.6.2. Clinical Trial Site Management Services
- 6.6.3. Recent Developments and Future Outlook
- 6.7. Worldwide Clinical Trials
- 6.7.1. Company Overview
- 6.7.2. Clinical Trial Site Management Services
- 6.7.3. Recent Developments and Future Outlook
7. COMPANY PROFILES: SITE MANAGEMENT ORGANIZATIONS IN EUROPE
- 7.1. Chapter Overview
- 7.2. CROMSOURCE
- 7.2.1. Company Overview
- 7.2.2. Clinical Trial Site Management Services
- 7.2.3. Recent Developments and Future Outlook
- 7.3. Fidelis Research
- 7.3.1. Company Overview
- 7.3.2. Clinical Trial Site Management Services
- 7.3.3. Recent Developments and Future Outlook
- 7.4. Scandinavian CRO
- 7.4.1. Company Overview
- 7.4.2. Clinical Trial Site Management Services
- 7.4.3. Recent Developments and Future Outlook
- 7.5. TFS HealthScience
- 7.5.1. Company Overview
- 7.5.2. Clinical Trial Site Management Services
- 7.5.3. Recent Developments and Future Outlook
- 7.6. Trialbee
- 7.6.1. Company Overview
- 7.6.2. Clinical Trial Site Management Services
- 7.6.3. Recent Developments and Future Outlook
8. COMPANY PROFILES: SITE MANAGEMENT ORGANIZATIONS IN ASIA-PACIFIC
- 8.1. Chapter Overview
- 8.2. CMIC Group
- 8.2.1. Company Overview
- 8.2.2. Clinical Trial Site Management Services
- 8.2.3. Recent Developments and Future Outlook
- 8.3. George Clinical
- 8.3.1. Company Overview
- 8.3.2. Clinical Trial Site Management Service
- 8.3.3. Recent Developments and Future Outlook
- 8.4. Tigermed
- 8.4.1. Company Overview
- 8.4.2. Clinical Trial Site Management Services
- 8.4.3. Recent Developments and Future Outlook
- 8.5. Veeda Clinical Research
- 8.5.1. Company Overview
- 8.5.2. Clinical Trial Site Management Services
- 8.5.3. Recent Developments and Future Outlook
9. PARTNERSHIPS AND COLLABORATIONS
- 9.1. Chapter Overview
- 9.2. Partnership Models
- 9.3. Site Management Organizations: Partnerships and Collaborations
- 9.3.1. Analysis by Year of Partnership
- 9.3.2. Analysis by Type of Partnership
- 9.3.3. Analysis by Year and Type of Partnership
- 9.3.4. Analysis by Focus Area
- 9.3.5. Most Active Players: Analysis by Number of Partnerships
- 9.3.6. Geographical Analysis
- 9.3.6.1. Region-wise Distribution
- 9.3.6.2. Country-wise Distribution
10. FUNDING AND INVESTMENT ANALYSIS
- 10.1. Chapter Overview
- 10.2. Types of Funding
- 10.3. Site Management Organizations: Funding and Investment Analysis
- 10.3.1. Analysis by Year of Investment
- 10.3.2. Analysis by Amount Invested
- 10.3.3. Analysis by Type of Funding
- 10.3.4. Year-wise Analysis by Type of Funding and Amount Invested
- 10.3.5. Most Active Players: Analysis by Number of Funding Instances
- 10.3.6. Most Active Investors: Analysis by Number of Funding Instances
- 10.3.7. Analysis by Type of Investor
- 10.3.8. Analysis by Geography
11. KEY INSIGHTS FROM CLINICAL TRIAL ANALYSIS
- 11.1. Chapter Overview
- 11.2. Scope and Methodology
- 11.3. Site Management Organizations: Clinical Trial Key Insights
- 11.3.1. Analysis by Trial Registration Year
- 11.3.2. Analysis by Trial Registration Year and Enrolled Patient Population
- 11.3.3. Analysis by Trial Status
- 11.3.4. Analysis by Trial Registration Year and Trial Status
- 11.3.5. Analysis by Trial Phase
- 11.3.6. Analysis by Trial Phase and Enrolled Patient Population
- 11.3.7. Analysis by Trial Registration Year and Trial Phase (in terms of Number of Clinical Trials)
- 11.3.8. Analysis by Trial Registration Year and Trial Phase (in terms of Number of Enrolled Patient Population)
- 11.3.9. Analysis by Type of Sponsor / Collaborator
- 11.3.10. Geographical Analysis by Number of Clinical Trials
- 11.3.11. Geographical Analysis by Enrolled Patient Population
12. ANALYSIS OF DEMAND FOR CLINICAL TRIAL PARTICIPANTS
- 12.1. Chapter Overview
- 12.2. Methodology and Key Assumptions
- 12.3. Global Demand for Clinical Trial Participants: Analysis by Enrolled Patient Population
- 12.3.1. Analysis of Demand by Trial Phase
- 12.3.2. Analysis of Demand by Therapeutic Area
- 12.3.3. Geographical Demand by Enrolled Patient Population
13. MARKET FORECAST AND OPPORTUNITY ANALYSIS
- 13.1. Chapter Overview
- 13.2. Forecast Methodology and Key Assumptions
- 13.3. Global Site Management Organizations Market, Till 2035
- 13.4. Site Management Organizations Market, 2021 and 2035: Distribution by Therapeutic Area
- 13.5. Site Management Organizations Market, Till 2035: Distribution by Trial Phase
- 13.6. Site Management Organizations Market, Till 2035: Distribution by Clinical Trial Components
- 13.7. Site Management Organizations Market, Till 2035: Distribution by Type of Intervention
- 13.8. Site Management Organizations Market, 2021 and 2035: Distribution by Region
- 13.8.1. Site Management Organizations Market, 2021 and 2035: Distribution by Country
- 13.8.2. Site Management Organizations Market in North America, Till 2035
- 13.8.2.1. Site Management Organizations Market in the US, Till 2035
- 13.8.2.2. Site Management Organizations Market in Canada, Till 2035
- 13.8.2.3. Site Management Organizations Market in Rest of North America, Till 2035
- 13.8.3. Site Management Organizations Market in Europe, Till 2035
- 13.8.3.1. Site Management Organizations Market in the UK, Till 2035
- 13.8.3.2. Site Management Organizations Market in France, Till 2035
- 13.8.3.3. Site Management Organizations Market in Germany, Till 2035
- 13.8.3.4. Site Management Organizations Market in Spain, Till 2035
- 13.8.3.5. Site Management Organizations Market in Italy, Till 2035
- 13.8.3.6. Site Management Organizations Market in Rest of Europe, Till 2035
- 13.8.4. Site Management Organizations Market in Asia-Pacific, Till 2035
- 13.8.4.1. Site Management Organizations Market in China, Till 2035
- 13.8.4.2. Site Management Organizations Market in Korea, Till 2035
- 13.8.4.3. Site Management Organizations Market in India, Till 2035
- 13.8.4.4. Site Management Organizations Market in Australia, Till 2035
- 13.8.4.5. Site Management Organizations Market in Japan, Till 2035
- 13.8.4.6. Site Management Organizations Market in Israel, Till 2035
- 13.8.4.7. Site Management Organizations Market in Rest of Asia-Pacific, Till 2035
- 13.8.5. Site Management Organizations Market in Latin America, Till 2035
- 13.8.6. Site Management Organizations Market in MENA, Till 2035
- 13.8.7. Site Management Organizations Market in Rest of the World, Till 2035
14. CONCLUDING REMARKS
15. EXECUTIVE INSIGHTS
- 15.1. Chapter Overview
- 15.2. Company A
- 15.2.1. Company Snapshot
- 15.2.2. Interview Transcript: Country Head-Clinical Operations
- 15.3. Company B
- 15.3.1. Company Snapshot
- 15.3.2. Interview Transcript: Medical Director and Operations Manager
- 15.4. Company C
- 15.4.1. Company Snapshot
- 15.4.2. Interview Transcript: Project Manager
16. APPENDIX 1: TABULATED DATA
17. APPENDIX 2: LIST OF COMPANIES AND ORGANIZATIONS
List of Tables
- Table 3.1 Comparison of SMOs and CROs
- Table 4.1 List of Site Management Organizations
- Table 4.2 Site Management Organizations: Information on Type of Site Management Service(s) offered
- Table 4.3 Site Management Organizations: Information on Therapeutic Expertise
- Table 6.1 Site Management Organizations: List of Profiled Companies (North America)
- Table 6.2 FOMAT Medical Research: Company Snapshot
- Table 6.3 FOMAT Medical Research: Clinical Trial Site Management Service Portfolio
- Table 6.4 FOMAT Medical Research: Recent Developments and Future Outlook
- Table 6.5 Parexel: Company Snapshot
- Table 6.6 Parexel: Clinical Trial Site Management Service Portfolio
- Table 6.7 Paraxel: Recent Developments and Future Outlook
- Table 6.8 Pharm-Olam: Company Snapshot
- Table 6.9 Pharm-Olam: Clinical Trial Site Management Service Portfolio
- Table 6.10 Pharm-Olam: Recent Developments and Future Outlook
- Table 6.11 Veristat: Company Snapshot
- Table 6.12 Veristat: Clinical Trial Site Management Service Portfolio
- Table 6.13 Veristat: Recent Developments and Future Outlook
- Table 6.14 WCCT Global: Company Snapshot
- Table 6.15 WCCT Global: Clinical Trial Site Management Service Portfolio
- Table 6.16 WCCT Global: Recent Developments and Future Outlook
- Table 7.1 Site Management Organizations: List of Profiled Companies (Europe)
- Table 7.2 CROMSOURCE: Company Snapshot
- Table 7.3 CROMSOURCE: Clinical Trial Site Management Service Portfolio
- Table 7.4 CROMSOURCE: Recent Developments and Future Outlook
- Table 7.5 FIDELIS RESEARCH: Company Snapshot
- Table 7.6 FIDELIS RESEARCH: Clinical Trial Site Management Service Portfolio
- Table 7.7 FIDELIS RESEARCH: Recent Developments and Future Outlook
- Table 7.8 Scandinavian CRO: Company Snapshot
- Table 7.9 Scandinavian CRO: Clinical Trial Site Management Service Portfolio
- Table 7.10 Scandinavian CRO: Recent Developments and Future Outlook
- Table 7.11 TFS HealthScience: Company Snapshot
- Table 7.12 TFS HealthScience: Clinical Trial Site Management Service Portfolio
- Table 7.13 TFS HealthScience: Recent Developments and Future Outlook
- Table 7.14 Trialbee: Company Snapshot
- Table 7.15 Trialbee: Clinical Trial Site Management Service Portfolio
- Table 7.16 Trialbee: Recent Developments and Future Outlook
- Table 8.1 Site Management Organizations: List of Profiled Companies (Asia-Pacific)
- Table 8.2 CMIC Group: Company Snapshot
- Table 8.3 CMIC Group: Clinical Trial Site Management Service Portfolio
- Table 8.4 CMIC Group: Recent Developments and Future Outlook
- Table 8.5 George Clinical: Company Snapshot
- Table 8.6 George Clinical: Clinical Trial Site Management Service Portfolio
- Table 8.7 George Clinical: Recent Developments and Future Outlook
- Table 8.8 Tigermed: Company Snapshot
- Table 8.9 Tigermed: Clinical Trial Site Management Service Portfolio
- Table 8.10 Tigermed: Recent Developments and Future Outlook
- Table 8.11 Veeda Clinical Research: Company Snapshot
- Table 8.12 Veeda Clinical Research: Clinical Trial Site Management Service Portfolio
- Table 8.13 Veeda Clinical Research: Recent Developments and Future Outlook
- Table 9.1 Site Management Organizations: List of Partnerships and Collaborations, Since 2016
- Table 10.1 Site Management Organizations: List of Funding and Investments, Since 2015
- Table 12.1 Global Demand for Clinical Trial Participants: Average Number of Patients Enrolled by Trial Phase
- Table 16.1 Site Management Organizations: Distribution by Year of Establishment
- Table 16.2 Site Management Organizations: Distribution by Company Size
- Table 16.3 Site Management Organizations: Distribution by Location of Headquarters (Region-wise)
- Table 16.4 Site Management Organizations: Distribution by Location of Headquarters (Country-wise)
- Table 16.5 Site Management Organizations: Distribution by Company Size and Location of Headquarters
- Table 16.6 Site Management Organizations: Distribution by Service(s) Offered
- Table 16.7 Site Management Organizations: Distribution by Location of Headquarters and Service(s) Offered
- Table 16.8 Site Management Organizations: Distribution by Therapeutic Expertise
- Table 16.9 Site Management Organizations: Distribution by Geographical Reach
- Table 16.10 Partnerships and Collaborations: Cumulative Year-wise Trend, Since 2016
- Table 16.11 Partnerships and Collaborations: Distribution by Type of Partnership
- Table 16.12 Partnerships and Collaborations: Year-wise Trend by Type of Partnership
- Table 16.13 Partnerships and Collaborations: Distribution by Type of Partnership, Since 2016
- Table 16.14 Partnerships and Collaborations: Distribution by Focus Area
- Table 16.15 Most Active Players: Distribution by Number of Partnerships
- Table 16.16 Partnerships and Collaborations: Distribution by Region
- Table 16.17 Partnerships and Collaborations: Distribution by Country
- Table 16.18 Funding and Investment Analysis: Cumulative Year-wise Trend, Since 2015
- Table 16.19 Funding and Investment Analysis: Cumulative Year-wise Trend by Amount Invested, Since 2015 (USD Million)
- Table 16.20 Funding and Investment Analysis: Distribution by Type of Funding
- Table 16.21 Funding and Investment Analysis: Distribution of Amount Invested by Type of Funding (USD Million)
- Table 16.22 Funding and Investment Analysis: Year-wise Distribution by Amount Invested and Type of Funding, Since 2015 (USD Million)
- Table 16.23 Most Active Players: Distribution by Number of Funding Instances
- Table 16.24 Most Active Investors: Distribution by Number of Funding Instances
- Table 16.25 Funding and Investment Analysis: Distribution by Type of Investor
- Table 16.26 Funding and Investment Analysis: Distribution of Amount Invested by Type of Investor (USD Million)
- Table 16.27 Funding and Investment Analysis: Distribution by Region (Continent-wise)
- Table 16.28 Funding and Investment Analysis: Distribution by Region (Country-wise)
- Table 10.29 Funding and Investment Analysis: Distribution of Amount Invested by Region (Country-wise) (USD Million)
- Table 16.30 Clinical Trial Key Insights: Cumulative Distribution by Trial Registration Year, Since 2016
- Table 16.31 Clinical Trial Key Insights: Distribution by Trial Registration Year and Enrolled Patient Population, Since 2016
- Table 16.32 Clinical Trial Key Insights: Distribution by Trial Status
- Table 16.33 Clinical Trial Key Insights: Distribution by Trial Registration Year and Trial Status, Since 2016
- Table 16.34 Clinical Trial Key Insights: Distribution of Number of Trials by Trial Phase
- Table 16.35 Clinical Trial Key Insights: Distribution of Enrolled Patient Population by Trial Phase
- Table 16.36 Clinical Trial Key Insights: Year-wise Distribution by Number of Trials and Trial Phase, Since 2016
- Table 16.37 Clinical Trial Key Insights: Year-wise Distribution by Number of Enrolled Patient Population and Trial Phase, Since 2016
- Table 16.38 Clinical Trial Key Insights: Distribution by Type of Sponsor / Collaborator
- Table 16.39 Clinical Trial Key Insights: Geographical Distribution by Number of Clinical Trials
- Table 16.40 Clinical Trial Key Insights: Geographical Distribution by Enrolled Patient Population
- Table 16.41 Global Demand for Clinical Trial Participants, Till 2035 (Million Patients)
- Table 16.42 Global Demand for Clinical Trial Participants: Distribution by Therapeutic Area (Million Patients)
- Table 16.43 Global Demand for Clinical Trial Participants: Distribution by Trial Phase (Million Patients)
- Table 16.44 Global Demand for Clinical Trial Participants: Distribution by Region (Million Patients)
- Table 16.45 Global Site Management Organizations Market, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
- Table 16.46 Site Management Organizations Market, 2021 and 2035: Distribution by Therapeutic Area (USD Billion)
- Table 16.47 Site Management Organizations Market, Till 2035: Distribution by Trial Phase (USD Billion)
- Table 16.48 Site Management Organizations Market, 2021: Distribution by Clinical Trial Components (USD Billion)
- Table 16.49 Site Management Organizations Market, Till 2035: Distribution by Type of Intervention (USD Billion)
- Table 16.50 Site Management Organizations Market, 2021 and 2035: Distribution by Region (USD Billion)
- Table 16.51 Site Management Organizations Market, 2021 and 2035: Distribution by Country (USD Billion)
- Table 16.52 Site Management Organizations Market in North America, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
- Table 16.53 Site Management Organizations Market in the US, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
- Table 16.54 Site Management Organizations Market in Canada, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
- Table 16.55 Site Management Organizations Market in Rest of North America, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
- Table 16.56 Site Management Organizations Market in Europe, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
- Table 16.57 Site Management Organizations Market in the UK, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
- Table 16.58 Site Management Organizations Market in France, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
- Table 16.59 Site Management Organizations Market in Germany, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
- Table 16.60 Site Management Organizations Market in Spain, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
- Table 16.61 Site Management Organizations Market in Italy, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
- Table 16.62 Site Management Organizations Market in Rest of Europe, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
- Table 16.63 Site Management Organizations Market in Asia-Pacific, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
- Table 16.64 Site Management Organizations Market in China, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
- Table 16.65 Site Management Organizations Market in Korea, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
- Table 16.66 Site Management Organizations Market in India, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
- Table 16.67 Site Management Organizations Market in Australia, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
- Table 16.68 Site Management Organizations Market in Japan, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
- Table 16.69 Site Management Organizations Market in Israel, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
- Table 16.70 Site Management Organizations Market in Rest of Asia-Pacific, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
- Table 16.71 Site Management Organizations Market in Latin America, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
- Table 16.72 Site Management Organizations Market in MENA, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
- Table 16.73 Site Management Organizations Market in Rest of the World, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
List of Figures
- Figure 2.1 Executive Summary: Market Forecast
- Figure 2.2 Executive Summary: Overall Market Landscape
- Figure 2.3 Executive Summary: Partnerships and Collaborations
- Figure 2.4 Executive Summary: Funding and Investments
- Figure 2.5 Executive Summary: Clinical Trial Key Insights
- Figure 2.6 Executive Summary: Analysis of Demand for Clinical Trial Participants
- Figure 3.1 Working Model of Site Management Organizations
- Figure 3.2 Services Offered by Site Management Organizations
- Figure 4.1 Site Management Organizations: Distribution by Year of Establishment
- Figure 4.2 Site Management Organizations: Distribution by Company Size
- Figure 4.3 Site Management Organizations: Distribution by Location of Headquarters (Region-wise)
- Figure 4.4 Site Management Organizations: Distribution by Location of Headquarters (Country-wise)
- Figure 4.5 Site Management Organizations: Distribution by Company Size and Location of Headquarters
- Figure 4.6 Site Management Organizations: Distribution by Service(s) Offered
- Figure 4.7 Site Management Organizations: Distribution by Location of Headquarters and Service(s) Offered
- Figure 4.8 Site Management Organizations: Distribution by Therapeutic Expertise
- Figure 4.9 Site Management Organizations: Distribution by Geographical Reach
- Figure 5.1 Company Competitiveness Analysis: Small Players in North America
- Figure 5.2 Company Competitiveness Analysis: Mid-sized Players in North America
- Figure 5.3 Company Competitiveness Analysis: Large Players in North America
- Figure 5.4 Company Competitiveness Analysis: Small Players in Europe
- Figure 5.5 Company Competitiveness Analysis: Mid-sized Players in Europe
- Figure 5.6 Company Competitiveness Analysis: Large Players in Europe
- Figure 5.7 Company Competitiveness Analysis: Small Players in Asia-Pacific and Rest of the World
- Figure 5.8 Company Competitiveness Analysis: Mid-sized Players in Asia-Pacific and Rest of the World
- Figure 5.9 Company Competitiveness Analysis: Large Players in Asia-Pacific and Rest of the World
- Figure 9.1 Partnerships and Collaborations: Cumulative Year-wise Trend, Since 2016
- Figure 9.2 Partnerships and Collaborations: Distribution by Type of Partnership
- Figure 9.3 Partnerships and Collaborations: Year-wise Trend by Type of Partnership
- Figure 9.4 Partnerships and Collaborations: Distribution by Type of Partnership, Since 2016
- Figure 9.5 Partnerships and Collaborations: Distribution by Focus Area
- Figure 9.6 Most Active Players: Distribution by Number of Partnerships
- Figure 9.7 Partnerships and Collaborations: Distribution by Region
- Figure 9.8 Partnerships and Collaborations: Distribution by Country
- Figure 10.1 Funding and Investment Analysis: Cumulative Year-wise Trend, Since 2015
- Figure 10.2 Funding and Investment Analysis: Cumulative Year-wise Trend by Amount Invested, Since 2015 (USD Million)
- Figure 10.3 Funding and Investment Analysis: Distribution by Type of Funding
- Figure 10.4 Funding and Investment Analysis: Distribution of Amount Invested by Type of Funding (USD Million)
- Figure 10.5 Funding and Investment Analysis: Year-wise Distribution by Amount Invested and Type of Funding, Since 2015 (USD Million)
- Figure 10.6 Most Active Players: Distribution by Number of Funding Instances
- Figure 10.7 Most Active Investors: Distribution by Number of Funding Instances
- Figure 10.8 Funding and Investment Analysis: Distribution by Type of Investor
- Figure 10.9 Funding and Investment Analysis: Distribution of Amount Invested by Type of Investor (USD Million)
- Figure 10.10 Funding and Investment Analysis: Distribution by Region (Continent-wise)
- Figure 10.11 Funding and Investment Analysis: Distribution by Region (Country-wise)
- Figure 10.12 Funding and Investment Analysis: Distribution of Amount Invested by Region (Country-wise) (USD Million)
- Figure 11.1 Clinical Trial Key Insights: Scope and Methodology
- Figure 11.2 Clinical Trial Key Insights: Cumulative Distribution by Trial Registration Year, Since 2016
- Figure 11.3 Clinical Trial Key Insights: Distribution by Trial Registration Year and Enrolled Patient Population, Since 2016
- Figure 11.4 Clinical Trial Key Insights: Distribution by Trial Status
- Figure 11.5 Clinical Trial Key Insights: Distribution by Trial Registration Year and Trial Status, Since 2016
- Figure 11.6 Clinical Trial Key Insights: Distribution of Number of Trials by Trial Phase
- Figure 11.7 Clinical Trial Key Insights: Distribution of Enrolled Patient Population by Trial Phase
- Figure 11.8 Clinical Trial Key Insights: Year-wise Distribution by Number of Trials and Trial Phase, Since 2016
- Figure 11.9 Clinical Trial Key Insights: Year-wise Distribution by Number of Enrolled Patient Population and Trial Phase, Since 2016
- Figure 11.10 Clinical Trial Key Insights: Distribution by Type of Sponsor / Collaborator
- Figure 11.11 Clinical Trial Key Insights: Geographical Distribution by Number of Clinical Trials
- Figure 11.12 Clinical Trial Key Insights: Geographical Distribution by Enrolled Patient Population
- Figure 12.1 Analysis of Demand for Clinical Trial Participants: Scope and Methodology
- Figure 12.2 Global Demand for Clinical Trial Participants, Till 2035 (Million Patients)
- Figure 12.3 Global Demand for Clinical Trial Participants, Till 2035: Distribution by Therapeutic Area (Million Patients)
- Figure 12.4 Global Demand for Clinical Trial Participants, Till 2035: Distribution by Trial Phase (Million Patients)
- Figure 12.5 Global Demand for Clinical Trial Participants, 2021: Distribution by Region (Million Patients)
- Figure 13.1 Global Site Management Organizations Market, Till 2035 (USD Billion)
- Figure 13.2 Site Management Organizations Market, 2021 and 2035: Distribution by Therapeutic Area (USD Billion)
- Figure 13.3 Site Management Organizations Market, Till 2035: Distribution by Trial Phase (USD Billion)
- Figure 13.4 Site Management Organizations Market, 2021: Distribution by Clinical Trial Components (USD Billion)
- Figure 13.5 Site Management Organizations Market, Till 2035: Distribution by Type of Intervention (USD Billion)
- Figure 13.6 Site Management Organizations Market, 2021 and 2035: Distribution by Region (USD Billion)
- Figure 13.7 Site Management Organizations Market, 2021 and 2035: Distribution by Country (USD Billion)
- Figure 13.8 Site Management Organizations Market in North America, Till 2035 (USD Billion)
- Figure 13.9 Site Management Organizations Market in the US, Till 2035 (USD Billion)
- Figure 13.10 Site Management Organizations Market in Canada, Till 2035 (USD Billion)
- Figure 13.11 Site Management Organizations Market in Rest of North America, Till 2035 (USD Billion)
- Figure 13.12 Site Management Organizations Market in Europe, Till 2035 (USD Billion)
- Figure 13.13 Site Management Organizations Market in the UK, Till 2035 (USD Billion)
- Figure 13.14 Site Management Organizations Market in France, Till 2035 (USD Billion)
- Figure 13.15 Site Management Organizations Market in Germany, Till 2035 (USD Billion)
- Figure 13.16 Site Management Organizations Market in Spain, Till 2035 (USD Billion)
- Figure 13.17 Site Management Organizations Market in Italy, Till 2035 (USD Billion)
- Figure 13.18 Site Management Organizations Market in Rest of Europe, Till 2035 (USD Billion)
- Figure 13.19 Site Management Organizations Market in Asia-Pacific, Till 2035 (USD Billion)
- Figure 13.20 Site Management Organizations Market in China, Till 2035 (USD Billion)
- Figure 13.21 Site Management Organizations Market in Korea, Till 2035 (USD Billion)
- Figure 13.22 Site Management Organizations Market in India, Till 2035 (USD Billion)
- Figure 13.23 Site Management Organizations Market in Australia, Till 2035 (USD Billion)
- Figure 13.24 Site Management Organizations Market in Japan, Till 2035 (USD Billion)
- Figure 13.25 Site Management Organizations Market in Israel, Till 2035 (USD Billion)
- Figure 13.26 Site Management Organizations Market in Rest of Asia-Pacific, Till 2035 (USD Billion)
- Figure 13.27 Site Management Organizations Market in Latin America, Till 2035 (USD Billion)
- Figure 13.28 Site Management Organizations Market in MENA, Till 2035 (USD Billion)
- Figure 13.29 Site Management Organizations Market in Rest of the World, Till 2035 (USD Billion)
- Figure 14.1 Concluding Remarks: Overall Market Landscape
- Figure 14.2 Concluding Remarks: Partnerships and Collaborations
- Figure 14.3 Concluding Remarks: Funding and Investment
- Figure 14.4 Concluding Remarks: Clinical Trial Key Insights
- Figure 14.5 Concluding Remarks: Analysis of Demand for Clinical Trial Participants
- Figure 14.6 Concluding Remarks: Market Forecast



