 |
市场调查报告书
商品编码
1666711
电子临床解决方案市场机会、成长动力、产业趋势分析与 2025 - 2034 年预测eClinical Solutions Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
2024 年全球电子临床解决方案市场价值为 111 亿美元,资料2025 年至 2034 年的复合年增长率为 12.1%。穿戴式装置、行动健康应用程式和电子健康记录的日益普及正在提高即时监控和资料准确性。
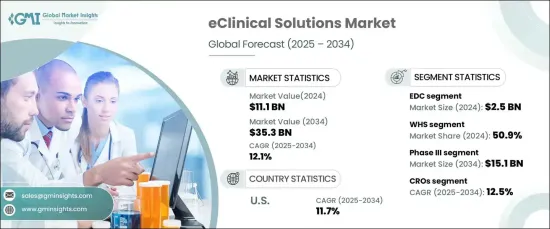
监管机构也在提倡使用数位化工具来优化试验效率并维护病患安全标准。此外,随着个人化医疗和慢性病治疗的进步,全球临床试验的数量不断增加,也增加了对先进的电子临床解决方案的需求。这些平台简化了患者招募、合规性监控和即时资料分析等复杂流程,使其对于管理日益复杂的大规模试验至关重要。
| 市场范围 | |
|---|---|
| 起始年份 | 2024 |
| 预测年份 | 2025-2034 |
| 起始值 | 111亿美元 |
| 预测值 | 353亿美元 |
| 复合年增长率 | 12.1% |
eClinical 解决方案包括旨在简化临床试验管理的先进软体和数位平台。这些工具整合了来自多个来源的资料,包括电子资料收集 (EDC) 系统、临床试验管理系统 (CTMS) 和电子病患报告结果 (ePRO),确保提高准确性和法规遵循。 EDC 部门在 2024 年引领市场,创造 25 亿美元的收入,因为它能够增强资料处理、最大限度地减少人工错误并加速即时监控。随着试验变得越来越复杂,对此类系统的需求持续上升。
2024 年,网页寄存解决方案占据了最大的市场份额,为 50.9%。这些解决方案支援远端存取、与其他数位工具的无缝整合以及强大的安全功能,从而推动了其广泛应用。他们促进即时协作和资料共享的能力进一步巩固了他们的市场地位。
2024 年,III 期临床试验领域占据市场主导地位,预计到 2034 年将达到 151 亿美元。 此阶段涉及大规模测试以评估治疗的安全性和有效性,因此需要高效的资料管理和监控。电子临床工具透过实现即时资料撷取和确保符合监管要求来增强这些流程。
预测期内,合约研究组织 (CRO) 的复合年增长率将达到 12.5%。由于 CRO 的专业知识和效率,将临床研究外包给 CRO 的趋势日益明显,成为市场的主要驱动力。这些组织利用 eClinical 解决方案来管理复杂的跨国试验、简化操作并确保即时资料分析。
2024 年美国将主导北美市场,预计将以 11.7% 的复合年增长率保持领先地位。这归功于该国强劲的製药和生物技术产业,以及对技术创新和分散试验的重视。主要研究机构和监管机构的存在进一步支持了先进的电子临床技术的采用。
目录
第 1 章:方法论与范围
第 2 章:执行摘要
第 3 章:产业洞察
- 产业生态系统分析
- 产业衝击力
- 成长动力
- 政府对临床试验的资助增加
- 将临床试验外包给 CRO 的需求不断增长
- 临床试验中越来越多采用软体解决方案
- 全球研发支出增加
- 产业陷阱与挑战
- 植入成本高
- 发展中国家对电子临床解决方案的采用率低且缺乏认识
- 成长动力
- 成长潜力分析
- 监管格局
- 软体格局
- 波特的分析
- PESTEL 分析
第四章:竞争格局
- 介绍
- 公司矩阵分析
- 主要市场参与者的竞争分析
- 竞争定位矩阵
- 策略仪表板
第 5 章:市场估计与预测:按解决方案,2021 年至 2034 年
- 主要趋势
- 随机化和试验供应管理
- 临床资料管理系统 (CDMS)
- 临床试验管理系统(CTMS)
- 电子临床结果评估 (eCOA)
- 电子审判主文件 (eTMF)
- 电子资料采集 (EDC)
- 其他解决方案
第六章:市场估计与预测:依交付方式,2021 – 2034 年
- 主要趋势
- 获得许可的企业(本地)解决方案
- 基于云端的(SAAS)解决方案(CBS)
- 网路託管(按需)解决方案 (WHS)
第 7 章:市场估计与预测:按临床试验阶段,2021 年至 2034 年
- 主要趋势
- 第一阶段
- 第二阶段
- 第三阶段
- 第四阶段
第 8 章:市场估计与预测:依最终用途,2021 年至 2034 年
- 主要趋势
- 合约研究组织 (CRO)
- 医疗器材公司
- 製药/生物技术公司
- 医院和诊所
- 其他最终用途
第 9 章:市场估计与预测:按地区,2021 年至 2034 年
- 主要趋势
- 北美洲
- 我们
- 加拿大
- 欧洲
- 德国
- 英国
- 法国
- 西班牙
- 义大利
- 波兰
- 瑞士
- 荷兰
- 亚太地区
- 中国
- 日本
- 印度
- 澳洲
- 韩国
- 印尼
- 泰国
- 拉丁美洲
- 巴西
- 墨西哥
- 阿根廷
- 阿根廷
- 智利
- 中东和非洲
- 南非
- 沙乌地阿拉伯
- 阿联酋
- 埃及
第十章:公司简介
- Acralyon
- Anju Software (OmniComm System)
- Bio-optronics
- Clario
- Dassault Systemes (Medidata)
- Datatrak
- Ennov
- IBM
- Medsharing
- Multihealth Group (Clinfile)
- OpenClinica
- Oracle Corporation
- Parexel International
- Signant Health (CRF Health)
- Veeva Systems
The Global EClinical Solutions Market, valued at USD 11.1 billion in 2024, is forecasted to grow at a CAGR of 12.1% from 2025 to 2034. This rapid growth is driven by the increasing adoption of decentralized clinical trials, which utilize digital technologies to streamline data collection and improve patient engagement. The rising use of wearable devices, mobile health applications, and electronic health records is enhancing real-time monitoring and data accuracy.

Regulatory bodies are also advocating for digital tools to optimize trial efficiency and maintain patient safety standards. Additionally, the escalating volume of clinical trials worldwide, fueled by advancements in personalized medicine and treatments for chronic conditions, is amplifying the need for advanced eClinical solutions. These platforms simplify complex processes such as patient recruitment, compliance monitoring, and real-time data analytics, making them essential in managing the growing intricacies of large-scale trials.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $11.1 Billion |
| Forecast Value | $35.3 Billion |
| CAGR | 12.1% |
eClinical solutions encompass advanced software and digital platforms designed to streamline clinical trial management. These tools integrate data from multiple sources, including electronic data capture (EDC) systems, clinical trial management systems (CTMS), and electronic patient-reported outcomes (ePRO), ensuring improved accuracy and regulatory compliance. The EDC segment led the market in 2024, generating USD 2.5 billion, due to its ability to enhance data processing, minimize manual errors, and accelerate real-time monitoring. As trials become more complex, the demand for such systems continues to rise.
Web-hosted solutions accounted for the largest market share of 50.9% in 2024. Their scalability, affordability, and ease of deployment make them ideal for decentralized trials. These solutions enable remote access, seamless integration with other digital tools, and robust security features, driving their widespread adoption. Their ability to facilitate real-time collaboration and data sharing has further solidified their market position.
The phase III clinical trial segment dominated the market in 2024, projected to reach USD 15.1 billion by 2034. This phase involves large-scale testing to evaluate treatment safety and efficacy, necessitating efficient data management and monitoring. eClinical tools enhance these processes by enabling real-time data capture and ensuring compliance with regulatory requirements.
Contract research organizations (CROs) are set to grow at a 12.5% CAGR over the forecast period. The rising trend of outsourcing clinical research to CROs, driven by their expertise and efficiency, is a key market driver. These organizations utilize eClinical solutions to manage complex multinational trials, streamline operations, and ensure real-time data analysis.
The US dominated the North American market in 2024 and is expected to maintain its lead with a CAGR of 11.7%. This is attributed to the country's robust pharmaceutical and biotechnology industries, as well as its emphasis on technological innovation and decentralized trials. The presence of major research institutions and regulatory bodies further supports the adoption of advanced eClinical technologies.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definition
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Base estimates and calculations
- 1.3.1 Base year calculation
- 1.3.2 Key trends for market estimation
- 1.4 Forecast model
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.5.2 Data mining sources
Chapter 2 Executive Summary
- 2.1 Industry 360° synopsis
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Rise in government funding grants for clinical trials
- 3.2.1.2 Growing demand for outsourcing clinical trials to CROs
- 3.2.1.3 Increasing adoption of software solutions in clinical trials
- 3.2.1.4 Increasing R&D expenditure across the globe
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 High implantation cost
- 3.2.2.2 Low adoption and lack of awareness pertaining to eClinical solutions in developing countries
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.5 Software landscape
- 3.6 Porter’s analysis
- 3.7 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company matrix analysis
- 4.3 Competitive analysis of major market players
- 4.4 Competitive positioning matrix
- 4.5 Strategy dashboard
Chapter 5 Market Estimates and Forecast, By Solution, 2021 – 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Randomization and trial supply management
- 5.3 Clinical data management system (CDMS)
- 5.4 Clinical trial management system (CTMS)
- 5.5 Electronic clinical outcome assessment (eCOA)
- 5.6 Electronic trial master file (eTMF)
- 5.7 Electronic data capture (EDC)
- 5.8 Other solutions
Chapter 6 Market Estimates and Forecast, By Delivery Mode, 2021 – 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Licensed enterprise (on-premise) solutions
- 6.3 Cloud-based (SAAS) solution (CBS)
- 6.4 Web-hosted (on-demand) solution (WHS)
Chapter 7 Market Estimates and Forecast, By Clinical Trial Phase, 2021 – 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Phase I
- 7.3 Phase II
- 7.4 Phase III
- 7.5 Phase IV
Chapter 8 Market Estimates and Forecast, By End Use, 2021 – 2034 ($ Mn)
- 8.1 Key trends
- 8.2 Contract research organizations (CROs)
- 8.3 Medical device companies
- 8.4 Pharma/biotech companies
- 8.5 Hospitals and clinics
- 8.6 Other end use
Chapter 9 Market Estimates and Forecast, By Region, 2021 – 2034 ($ Mn)
- 9.1 Key trends
- 9.2 North America
- 9.2.1 U.S.
- 9.2.2 Canada
- 9.3 Europe
- 9.3.1 Germany
- 9.3.2 UK
- 9.3.3 France
- 9.3.4 Spain
- 9.3.5 Italy
- 9.3.6 Poland
- 9.3.7 Switzerland
- 9.3.8 Netherlands
- 9.4 Asia Pacific
- 9.4.1 China
- 9.4.2 Japan
- 9.4.3 India
- 9.4.4 Australia
- 9.4.5 South Korea
- 9.4.6 Indonesia
- 9.4.7 Thailand
- 9.5 Latin America
- 9.5.1 Brazil
- 9.5.2 Mexico
- 9.5.3 Argentina
- 9.5.4 Argentina
- 9.5.5 Chile
- 9.6 Middle East and Africa
- 9.6.1 South Africa
- 9.6.2 Saudi Arabia
- 9.6.3 UAE
- 9.6.4 Egypt
Chapter 10 Company Profiles
- 10.1 Acralyon
- 10.2 Anju Software (OmniComm System)
- 10.3 Bio-optronics
- 10.4 Clario
- 10.5 Dassault Systemes (Medidata)
- 10.6 Datatrak
- 10.7 Ennov
- 10.8 IBM
- 10.9 Medsharing
- 10.10 Multihealth Group (Clinfile)
- 10.11 OpenClinica
- 10.12 Oracle Corporation
- 10.13 Parexel International
- 10.14 Signant Health (CRF Health)
- 10.15 Veeva Systems











