 |
市场调查报告书
商品编码
1684650
CAR-T 细胞疗法市场机会、成长动力、产业趋势分析及 2025 - 2034 年预测CAR T-cell Therapy Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
2024 年全球 CAR-T 细胞疗法市场价值为 43 亿美元,预计将经历强劲的成长轨迹,2025 年至 2034 年的复合年增长率将达到 30.5%。嵌合抗原受体 (CAR) T 细胞疗法正在彻底改变癌症治疗,标誌着肿瘤学的一个重要里程碑。这种先进的免疫疗法涉及对患者 T 细胞进行基因改造,以便更好地瞄准和消灭癌细胞。 CAR T 细胞疗法不仅为患者带来了新的希望,也改变了我们治疗某些癌症的方式。随着技术的日益普及和进步,这个市场有望不断扩张。
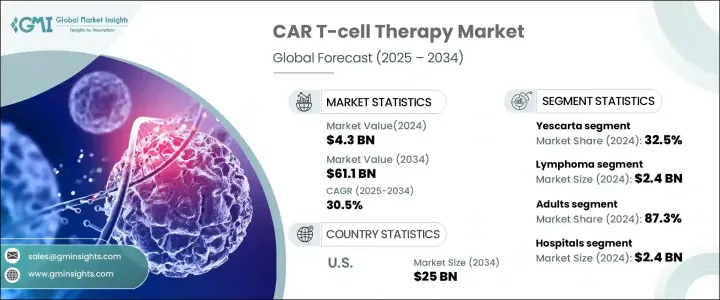
全球市场分为几种主要产品,包括 Abecma、Breyanzi、Carvykti、Kymriah、Tecartus、Yescarta 等。其中,Yescarta脱颖而出,成为市场领导者,2024年占了32.5%的市占率。 Yescarta占据市场主导地位的主要原因在于它对B细胞淋巴瘤患者已被证明有效,特别是在传统治疗方法往往无效的复发或难治性病例中。随着越来越多的患者寻求治疗恶性癌症的替代疗法,对 Yescarta 的需求持续增长,进一步巩固了其在该领域的领先地位。
| 市场范围 | |
|---|---|
| 起始年份 | 2024 |
| 预测年份 | 2025-2034 |
| 起始值 | 43亿美元 |
| 预测值 | 611亿美元 |
| 复合年增长率 | 30.5% |
从适应症来看,CAR-T 细胞治疗市场分为白血病、淋巴瘤、多发性骨髓瘤等,其中淋巴瘤的收入最高,2024 年将达到 24 亿美元。瀰漫性大 B 细胞淋巴瘤 (DLBCL) 是一种常见的非何杰金氏淋巴瘤,是这一增长的主要贡献者。 DLBCL 在接受传统疗法治疗后復发率较高,这推动了对 CAR T 细胞疗法的需求,这种疗法在针对和治疗侵袭性淋巴瘤方面表现出了显着的疗效。因此,CAR-T 细胞疗法越来越被视为治疗这些棘手癌症类型的重要解决方案,为别无选择的患者带来了新的希望。
预计到 2034 年,美国 CAR-T 细胞疗法市场规模将达到 250 亿美元,在支持性监管框架的推动下,市场将实现强劲成长。美国食品药物管理局(FDA)在 CAR-T 细胞疗法的快速开发和商业化中发挥了关键作用,透过突破性疗法认定、孤儿药地位和快速通道等加速审批流程提供关键的监管支持。这些措施加速了CAR-T细胞疗法的普及,确保需要救命治疗的患者能够及时获得治疗。
目录
第 1 章:方法论与范围
第 2 章:执行摘要
第 3 章:产业洞察
- 产业生态系统分析
- 产业衝击力
- 成长动力
- 人口中癌症恶性肿瘤病例不断增加
- 针对性治疗倾向日益增强
- 拥有强大的产品线,已获得跨地区监管部门的批准
- 产业陷阱与挑战
- 药品成本高阻碍市场成长
- CAR-T 细胞疗法的副作用
- 成长动力
- 成长潜力分析
- 监管格局
- 技术格局
- 未来市场趋势
- 差距分析
- 波特的分析
- PESTEL 分析
第四章:竞争格局
- 介绍
- 公司市占率分析
- 公司矩阵分析
- 主要市场参与者的竞争分析
- 竞争定位矩阵
- 策略仪表板
第 5 章:市场估计与预测:按产品,2021 年至 2034 年
- 主要趋势
- Abecma(idecabtagene vicleucel)
- Breyanzi(lisocabtagene maraleucel)
- Carvykti(ciltacabtagene autoleucel)
- Kymriah(tisagenlecleucel)
- Tecartus(brexucabtagene autoleucel)
- Yescarta(axicabtagene ciloleucel)
- 其他产品
第 6 章:市场估计与预测:按适应症,2021 年至 2034 年
- 主要趋势
- 白血病
- 淋巴瘤
- 多发骨髓瘤
- 其他适应症
第 7 章:市场估计与预测:按人口统计,2021 年至 2034 年
- 主要趋势
- 成年人
- 儿科
第 8 章:市场估计与预测:依最终用途,2021 年至 2034 年
- 主要趋势
- 医院
- 癌症治疗中心
- 专科诊所
第 9 章:市场估计与预测:按地区,2021 年至 2034 年
- 主要趋势
- 北美洲
- 我们
- 加拿大
- 欧洲
- 德国
- 英国
- 法国
- 西班牙
- 义大利
- 荷兰
- 亚太地区
- 中国
- 日本
- 印度
- 澳洲
- 韩国
- 拉丁美洲
- 巴西
- 墨西哥
- 阿根廷
- 中东和非洲
- 南非
- 沙乌地阿拉伯
- 阿联酋
第十章:公司简介
- Allogene Therapeutics
- Autolus Therapeutics
- bluebird bio
- Bristol-Myers Squibb Company
- CRISPR Therapeutics
- Gilead Sciences
- GSK plc.
- ImmunoAct
- Johnson & Johnson
- JW Therapeutics (Shanghai)
- Medigene AG
- Merck KGaA
- Novartis AG
- Sangamo Therapeutics
- Sorrento Therapeutics
The Global CAR T-Cell Therapy Market, valued at USD 4.3 billion in 2024, is projected to experience a robust growth trajectory, with an impressive CAGR of 30.5% from 2025 to 2034. Chimeric Antigen Receptor (CAR) T-cell therapy is revolutionizing cancer treatment, marking a significant milestone in oncology. This advanced form of immunotherapy involves the genetic modification of a patient's T-cells to better target and eliminate cancer cells. CAR T-cell therapy is not only offering new hope for patients but also changing the way we approach the treatment of certain cancers. With its growing adoption and advancements in technology, this market is poised for continuous expansion.

The global market is divided into several key products, including Abecma, Breyanzi, Carvykti, Kymriah, Tecartus, Yescarta, and others. Among these, Yescarta stands out as the market leader, capturing 32.5% of the total market share in 2024. The primary reason for Yescarta's market dominance lies in its proven effectiveness for patients with B-cell lymphomas, particularly in relapsed or refractory cases where traditional treatments often fall short. As more patients seek alternatives for aggressive forms of cancer, the demand for Yescarta continues to grow, further cementing its leadership in the space.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $4.3 Billion |
| Forecast Value | $61.1 Billion |
| CAGR | 30.5% |
In terms of indications, the CAR T-cell therapy market is categorized into leukemia, lymphoma, multiple myeloma, and others, with lymphoma generating the highest revenue of USD 2.4 billion in 2024. Diffuse large B-cell lymphoma (DLBCL), a common form of non-Hodgkin lymphoma is a major contributor to this growth. DLBCL's high relapse rates following conventional therapies have driven demand for CAR T-cell treatments, which have shown remarkable efficacy in targeting and treating aggressive lymphoma. As a result, CAR T-cell therapies are increasingly viewed as a vital solution in managing these challenging cancer types, offering new hope to patients with few options left.
The U.S. CAR T-cell therapy market is expected to reach USD 25 billion by 2034, with strong growth fueled by supportive regulatory frameworks. The U.S. Food and Drug Administration (FDA) has played a key role in the rapid development and commercialization of CAR T-cell therapies, providing critical regulatory support through expedited approval processes such as Breakthrough Therapy Designations, Orphan Drug status, and Fast Track pathways. These initiatives have fast-tracked the availability of CAR T-cell therapies, ensuring timely access for patients in need of life-saving treatments.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Base estimates and calculations
- 1.3.1 Base year calculation
- 1.3.2 Key trends for market estimation
- 1.4 Forecast model
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.5.2 Data mining sources
Chapter 2 Executive Summary
- 2.1 Industry 360° synopsis
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Growing cases of cancer malignancies across population
- 3.2.1.2 Rising inclination towards targeted treatment
- 3.2.1.3 Robust product pipeline with regulatory approvals across geographies
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 High drug cost impedes the market growth
- 3.2.2.2 Side-effects of CAR T-cell therapy
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.5 Technological landscape
- 3.6 Future market trends
- 3.7 Gap analysis
- 3.8 Porter's analysis
- 3.9 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.3 Company matrix analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Strategy dashboard
Chapter 5 Market Estimates and Forecast, By Product, 2021 – 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Abecma (idecabtagene vicleucel)
- 5.3 Breyanzi (lisocabtagene maraleucel)
- 5.4 Carvykti (ciltacabtagene autoleucel)
- 5.5 Kymriah (tisagenlecleucel)
- 5.6 Tecartus (brexucabtagene autoleucel)
- 5.7 Yescarta (axicabtagene ciloleucel)
- 5.8 Other products
Chapter 6 Market Estimates and Forecast, By Indication, 2021 – 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Leukemia
- 6.3 Lymphoma
- 6.4 Multiple myeloma
- 6.5 Other indications
Chapter 7 Market Estimates and Forecast, By Demographic, 2021 – 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Adults
- 7.3 Pediatric
Chapter 8 Market Estimates and Forecast, By End Use, 2021 – 2034 ($ Mn)
- 8.1 Key trends
- 8.2 Hospitals
- 8.3 Cancer treatment centers
- 8.4 Specialty clinics
Chapter 9 Market Estimates and Forecast, By Region, 2021 – 2034 ($ Mn)
- 9.1 Key trends
- 9.2 North America
- 9.2.1 U.S.
- 9.2.2 Canada
- 9.3 Europe
- 9.3.1 Germany
- 9.3.2 UK
- 9.3.3 France
- 9.3.4 Spain
- 9.3.5 Italy
- 9.3.6 Netherlands
- 9.4 Asia Pacific
- 9.4.1 China
- 9.4.2 Japan
- 9.4.3 India
- 9.4.4 Australia
- 9.4.5 South Korea
- 9.5 Latin America
- 9.5.1 Brazil
- 9.5.2 Mexico
- 9.5.3 Argentina
- 9.6 Middle East and Africa
- 9.6.1 South Africa
- 9.6.2 Saudi Arabia
- 9.6.3 UAE
Chapter 10 Company Profiles
- 10.1 Allogene Therapeutics
- 10.2 Autolus Therapeutics
- 10.3 bluebird bio
- 10.4 Bristol-Myers Squibb Company
- 10.5 CRISPR Therapeutics
- 10.6 Gilead Sciences
- 10.7 GSK plc.
- 10.8 ImmunoAct
- 10.9 Johnson & Johnson
- 10.10 JW Therapeutics (Shanghai)
- 10.11 Medigene AG
- 10.12 Merck KGaA
- 10.13 Novartis AG
- 10.14 Sangamo Therapeutics
- 10.15 Sorrento Therapeutics













