 |
市场调查报告书
商品编码
1766257
麻疹、腮腺炎、德国麻疹疫苗市场机会、成长动力、产业趋势分析及 2025 - 2034 年预测Measles, Mumps, Rubella Vaccine Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
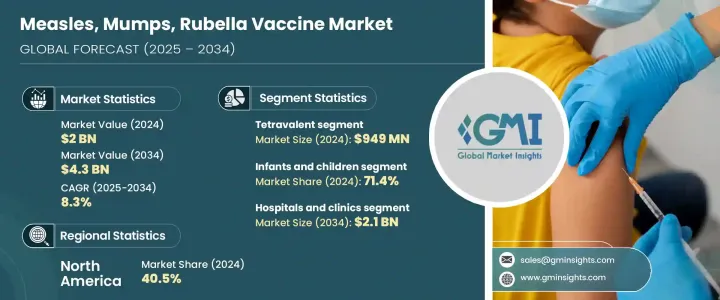
2024年,全球麻疹、腮腺炎和德国麻疹疫苗市场规模达20亿美元,预计2034年将以8.3%的复合年增长率成长,达到43亿美元。这一增长可归因于多种因素,包括对儿童免疫接种的日益重视、疫情爆发的增加以及全球卫生倡议的广泛开展。在中低收入国家,这个问题依然紧迫,由于资源有限且资金不足,需要有针对性的疫苗接种计画。此外,政府加大对旨在实现群体免疫的大规模疫苗接种活动的投入,也显着促进了市场发展,尤其是在发展中地区。
联合疫苗和耐热疫苗的普及减少了对冷链基础设施的依赖,从而提高了偏远地区的疫苗可及性,并提升了整体免疫效果。疫苗接种意识的提高、医疗基础设施的改善以及更具包容性的国家免疫计划,正在推动全球免疫接种率的提高。此外,新兴经济体出生率的上升以及麻腮风(MMR)疫苗被纳入常规疫苗接种计划,也扩大了市场的目标人口。旨在更好地控制疾病的增强型三价和四价疫苗的持续研发,也进一步支撑了市场的成长。
| 市场范围 | |
|---|---|
| 起始年份 | 2024 |
| 预测年份 | 2025-2034 |
| 起始值 | 20亿美元 |
| 预测值 | 43亿美元 |
| 复合年增长率 | 8.3% |
四价疫苗市场规模在2024年达到9.49亿美元。四价疫苗将多种疫苗合而为一,因其简化了疫苗接种程序并提高了依从性(尤其是在儿童中)而日益受到欢迎。由于四价疫苗保护范围更广且更容易接种,目前已被纳入全球各国(包括已开发经济体和新兴经济体)的国家免疫计画。四价疫苗不仅能预防麻疹和德国麻疹,还能预防水痘,这推动了MMRV疫苗的需求。冷链物流的改进以及世界卫生组织等全球卫生组织的支持也促进了四价疫苗在全球的普及,使其成为市场扩张的关键驱动力。
预计到2034年,医院和诊所市场规模将达到21亿美元。医院和诊所在麻腮风(MMR)疫苗市场中占据主导地位,这主要归功于它们积极参与免疫接种活动并提供丰富的儿科服务。这些医疗机构凭藉着完善的冷链基础设施和经验丰富的专业人员,成为城市和半城市地区疫苗接种的关键中心,确保疫苗的妥善储存和配送。此外,在疫情爆发期间,许多国家都依赖医院作为大规模免疫接种计画的主要枢纽,这进一步提升了该领域的市场份额。新兴市场中初级医疗保健中心数量的不断增加以及公私合作的不断加强,也促进了该领域的成长。
2024年,美国麻疹、腮腺炎、德国麻疹疫苗市场规模达7.261亿美元。儘管该市场已成熟且受到严格监管,但其发展仍受到公共卫生建议、学校免疫接种强制要求以及局部疫情应对活动的驱动。儘管疫苗接种犹豫不决偶尔会造成一些挫折,但在政府资金、价格调控以及儿童疫苗接种计划(VFC)等项目的支持下,儿童疫苗接种率依然保持高位。美国在疫苗研发和出口领域也继续发挥主导作用,进一步增强了其在全球免疫接种工作中的影响力。
全球麻疹、腮腺炎和德国麻疹疫苗市场的公司采取的关键策略包括利用疫苗传递技术进步,例如开发联合疫苗和低剂量疫苗,以提高患者的依从性。他们也致力于透过增强冷链基础设施来提高疫苗的可及性,并与全球卫生组织和政府建立强有力的合作,以确保疫苗能够覆盖服务匮乏的地区。此外,他们还投资研发,推出更新、更有效的疫苗配方,以提供更广泛的传染病防护,从而增强其市场占有率和长期可持续性。
目录
第一章:方法论与范围
第二章:执行摘要
第三章:行业洞察
- 产业生态系统分析
- 供应商格局
- 每个阶段的增值
- 影响价值链的因素
- 产业衝击力
- 成长动力
- 透过国家疫苗接种计划提高免疫覆盖率
- 发展中经济体儿科人口不断增加
- 疫苗配方和生产效率的进步
- 产业陷阱与挑战
- 严格的监管审批流程
- 偏远地区疫苗接种机会有限
- 市场机会
- 扩大疫苗分发的公私部门合作伙伴关係
- 联合疫苗需求不断增加,以减轻注射负担
- 成长动力
- 成长潜力分析
- 监管格局
- 北美洲
- 欧洲
- 亚太地区
- 波特的分析
- PESTEL分析
- 技术和创新格局
- 当前的技术趋势
- 新兴技术
- 价格趋势
- 按地区
- 按疫苗类型
- 未来市场趋势
- 技术和创新格局
- 当前的技术趋势
- 新兴技术
- 贸易统计(HS编码)
- 主要进口国
- 主要出口国
第四章:竞争格局
- 介绍
- 公司市占率分析
- 公司矩阵分析
- 主要市场参与者的竞争分析
- 竞争定位矩阵
- 关键进展
- 併购
- 伙伴关係和合作
- 新产品发布
- 扩张计划
第五章:市场估计与预测:按疫苗类型,2021-2034
- 主要趋势
- 单价
- 三价疫苗(MMR联合疫苗)
- 四价
第六章:市场估计与预测:按目标族群,2021-2034 年
- 主要趋势
- 婴儿和儿童
- 成年人
第七章:市场估计与预测:依最终用途,2021-2034
- 主要趋势
- 医院和诊所
- 政府和公共卫生计划
- 其他最终用途
第八章:市场估计与预测:按地区,2021-2034
- 主要趋势
- 北美洲
- 我们
- 加拿大
- 欧洲
- 德国
- 英国
- 法国
- 西班牙
- 义大利
- 荷兰
- 亚太地区
- 中国
- 日本
- 印度
- 澳洲
- 韩国
- 拉丁美洲
- 巴西
- 墨西哥
- 阿根廷
- 中东和非洲
- 南非
- 沙乌地阿拉伯
- 阿联酋
第九章:公司简介
- Actiza Pharmaceutical Pvt. Ltd.
- Beijing Institute of Biological Products
- Biological E
- GSK
- Merck
- Minhai Bio
- Sanofi
- Serum Institute of India
- Shanghai Institute of Biological Products
- Sinovac
- Zydus Life Science
The Global Measles, Mumps, Rubella Vaccine Market was valued at USD 2 billion in 2024 and is estimated to grow at a CAGR of 8.3% to reach USD 4.3 billion by 2034. This expansion can be attributed to several factors, including the growing emphasis on childhood immunization, a rise in outbreaks, and widespread global health initiatives. The issue remains pressing in lower-middle-income nations, where limited resources and inadequate funding necessitate targeted vaccination programs. Furthermore, the increasing government investment in mass vaccination drives aimed at achieving herd immunity is significantly boosting the market, especially in developing regions.

The availability of combination vaccines and thermostable vaccines, which reduce dependence on cold-chain infrastructure, is enhancing vaccine accessibility in remote areas, improving overall efficacy. Rising vaccination awareness, better healthcare infrastructure, and more inclusive national immunization programs are contributing to higher immunization rates globally. Additionally, the increasing birth rates in emerging economies and the integration of MMR vaccines into routine vaccination schedules are expanding the market's target demographic. The ongoing development of enhanced trivalent and quadrivalent vaccines, designed for better disease control, is further supporting market growth.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $2 Billion |
| Forecast Value | $4.3 Billion |
| CAGR | 8.3% |
The tetravalent segment was valued at USD 949 million in 2024. Tetravalent vaccines, which combine multiple vaccinations into one shot, are gaining popularity as they simplify vaccination schedules and improve compliance, particularly in children. These vaccines are now being integrated into national immunization programs worldwide, both in developed and emerging economies, as they offer broader protection and are easier to administer. Their ability to address not only measles and rubella but also varicella, is driving demand for MMRV formulations. Improved cold-chain logistics and support from global health organizations like the WHO are also contributing to the increased adoption of tetravalent vaccines globally, making this segment a key driver of market expansion.
The hospitals and clinics segment is projected to reach USD 2.1 billion by 2034. Hospitals and clinics maintain a dominant role in the MMR vaccine market, largely due to their involvement in immunization campaigns and the range of pediatric services they offer. These healthcare facilities are key centers for vaccine administration in both urban and semi-urban areas, thanks to their well-established cold chain infrastructure and skilled personnel who ensure proper storage and delivery of vaccines. Moreover, during epidemic outbreaks, many nations rely on hospitals as primary hubs for mass immunization programs, further boosting this segment's market share. The expanding number of primary healthcare centers and increasing public-private partnerships in emerging markets also contribute to the growth of this segment.
United States Measles, Mumps, Rubella Vaccine Market was valued at USD 726.1 million in 2024. While the market is mature and heavily regulated, it is still driven by public health recommendations, school immunization mandates, and campaigns responding to localized outbreaks. Although vaccine hesitancy has caused occasional setbacks, pediatric vaccination rates remain high, supported by government funding, controlled pricing, and programs like Vaccines for Children (VFC). The U.S. also continues to play a leading role in vaccine development and exportation, reinforcing its influence on global immunization efforts.
Key strategies adopted by companies in the Global Measles, Mumps, Rubella Vaccine Market include leveraging technological advancements in vaccine delivery, such as the development of combination vaccines and those requiring fewer doses, to increase patient compliance. They also focus on improving accessibility through enhanced cold-chain infrastructure and establishing strong collaborations with global health organizations and governments to ensure vaccines reach underserved regions. Moreover, they invest in research and development to introduce newer, more effective vaccine formulations that provide broader protection against infectious diseases, enhancing both their market presence and long-term sustainability.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definition
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/Country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
- 2.2 Key market trends
- 2.2.1 Regional
- 2.2.2 Vaccines type
- 2.2.3 Targeted population
- 2.2.4 End use
- 2.3 CXO perspectives: Strategic imperatives
- 2.3.1 Key decision points for industry executives
- 2.3.2 Critical success factors for market players
- 2.4 Future outlook and strategic recommendations
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.1.1 Supplier landscape
- 3.1.2 Value addition at each stage
- 3.1.3 Factor affecting the value chain
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Rising immunization coverage through national vaccination programs
- 3.2.1.2 Increasing pediatric population in developing economies
- 3.2.1.3 Advancements in vaccine formulation and production efficiency
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 Stringent regulatory approval processes
- 3.2.2.2 Limited access to vaccines in remote regions
- 3.2.3 Market opportunities
- 3.2.3.1 Expanding public-private partnerships for vaccine distribution
- 3.2.3.2 Growing demand for combination vaccines to reduce injection burden
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.4.1 North America
- 3.4.2 Europe
- 3.4.3 Asia Pacific
- 3.5 Porter's analysis
- 3.6 PESTEL analysis
- 3.7 Technology and innovation landscape
- 3.7.1 Current technological trends
- 3.7.2 Emerging technologies
- 3.8 Price trends
- 3.8.1 By region
- 3.8.2 By vaccines type
- 3.9 Future market trends
- 3.10 Technology and innovation landscape
- 3.10.1 Current technological trends
- 3.10.2 Emerging technologies
- 3.11 Trade statistics (HS code)
- 3.11.1 Major importing countries
- 3.11.2 Major exporting countries
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.3 Company matrix analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Key developments
- 4.6.1 Mergers and acquisitions
- 4.6.2 Partnerships and collaborations
- 4.6.3 New product launches
- 4.6.4 Expansion plans
Chapter 5 Market Estimates and Forecast, By Vaccines Type, 2021-2034 ($ Mn)
- 5.1 Key trends
- 5.2 Monovalent
- 5.3 Trivalent (combined MMR)
- 5.4 Tetravalent
Chapter 6 Market Estimates and Forecast, By Targeted Population, 2021-2034 ($ Mn)
- 6.1 Key trends
- 6.2 Infants and children
- 6.3 Adults
Chapter 7 Market Estimates and Forecast, By End Use, 2021-2034 ($ Mn)
- 7.1 Key trends
- 7.2 Hospitals and clinics
- 7.3 Government and public health programs
- 7.4 Other end use
Chapter 8 Market Estimates and Forecast, By Region, 2021-2034 ($ Mn)
- 8.1 Key trends
- 8.2 North America
- 8.2.1 U.S.
- 8.2.2 Canada
- 8.3 Europe
- 8.3.1 Germany
- 8.3.2 UK
- 8.3.3 France
- 8.3.4 Spain
- 8.3.5 Italy
- 8.3.6 Netherlands
- 8.4 Asia Pacific
- 8.4.1 China
- 8.4.2 Japan
- 8.4.3 India
- 8.4.4 Australia
- 8.4.5 South Korea
- 8.5 Latin America
- 8.5.1 Brazil
- 8.5.2 Mexico
- 8.5.3 Argentina
- 8.6 Middle East and Africa
- 8.6.1 South Africa
- 8.6.2 Saudi Arabia
- 8.6.3 UAE
Chapter 9 Company Profiles
- 9.1 Actiza Pharmaceutical Pvt. Ltd.
- 9.2 Beijing Institute of Biological Products
- 9.3 Biological E
- 9.4 GSK
- 9.5 Merck
- 9.6 Minhai Bio
- 9.7 Sanofi
- 9.8 Serum Institute of India
- 9.9 Shanghai Institute of Biological Products
- 9.10 Sinovac
- 9.11 Zydus Life Science







