 |
市场调查报告书
商品编码
1892872
家用睡眠呼吸中止症检测设备市场机会、成长驱动因素、产业趋势分析及预测(2026-2035年)Home Sleep Apnea Testing Devices Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2026 - 2035 |
||||||
2025 年全球家用睡眠呼吸中止症检测设备市场价值为 16 亿美元,预计到 2035 年将以 11.6% 的复合年增长率增长至 50 亿美元。
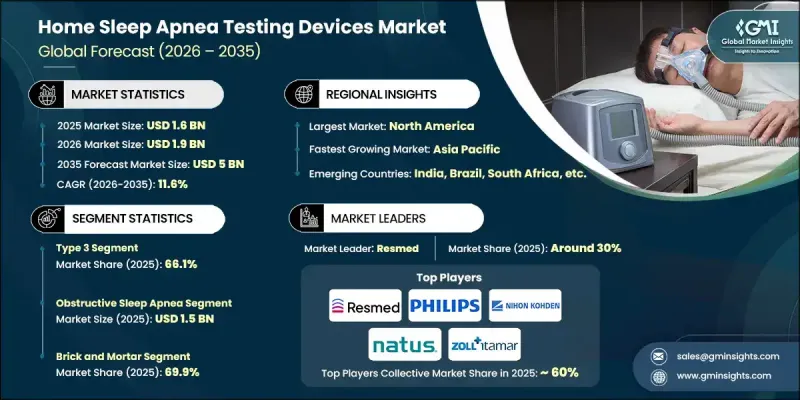
市场成长主要得益于老年人口的不断扩大、睡眠呼吸中止症及相关慢性疾病发病率的上升,以及便携式和穿戴式诊断技术的快速创新。大众对睡眠健康以及未确诊睡眠障碍相关临床风险的日益关注,也进一步推动了市场需求。居家检测方案因其价格实惠、方便快捷,且能够在传统临床环境之外提供可靠的诊断结果,正逐渐受到青睐。远距医疗和远距患者监测的日益普及也加速了这一趋势,因为这些设备能够轻鬆整合到数位化医疗工作流程中。肥胖、糖尿病和心血管疾病的日益流行,使得早期筛检和介入的需求日益增长,因为未经治疗的睡眠呼吸中止症会显着增加严重併发症的风险。感测器精度、无线资料传输和人工智慧技术的进步,使得设备更加小型化、精准化,并简化了患者独立操作的流程。这些因素使得居家睡眠呼吸中止症检测成为全球医疗保健系统中实用的第一线诊断工具。
| 市场范围 | |
|---|---|
| 起始年份 | 2025 |
| 预测年份 | 2026-2035 |
| 起始值 | 16亿美元 |
| 预测值 | 50亿美元 |
| 复合年增长率 | 11.6% |
2025年,3型设备市占率达66.1%。该细分市场的成长得益于对早期诊断和预防性护理的日益重视。这些设备能够追踪多种生理讯号,并在临床可靠性和使用者便利性之间取得平衡,因此适用于识别非复杂病例中的中度至重度阻塞性疾病。
2025 年,阻塞性睡眠呼吸中止症市场规模预计将达到 15 亿美元。这种疾病是睡眠呼吸中止症中最常见的类型,并且与代谢和心血管风险密切相关,因此及时诊断和监测至关重要。
预计2025年,北美家用睡眠呼吸中止症检测设备市场占有率将达到33.6%,并维持强劲成长。人们对睡眠障碍的高度关注、远距医疗的广泛应用、先进的医疗基础设施以及生活方式相关风险因素的高发生率,将继续推动该地区的需求成长。
目录
第一章:方法论与范围
第二章:执行概要
第三章:行业洞察
- 产业生态系分析
- 产业影响因素
- 成长驱动因素
- 睡眠呼吸中止症及其相关併发症的盛行率日益增加
- 提高人们对睡眠健康和居家睡眠呼吸中止症检测的认识
- 人口老化加剧
- 穿戴式和可携式HSAT设备的技术进步
- 产业陷阱与挑战
- 诊断设备需经过严格的监管审查。
- 与实验室多导睡眠图相比,准确度有限
- 机会
- 开发人工智慧驱动的诊断演算法
- 提供HSAT服务的远距医疗平台发展迅速
- 成长驱动因素
- 成长潜力分析
- 监管环境
- 北美洲
- 欧洲
- 亚太地区
- 拉丁美洲
- 技术与创新格局
- 当前技术趋势
- 新兴技术
- 价值链分析
- 报销方案
- 政策环境
- 消费者行为洞察
- 波特的分析
- PESTEL 分析
- 差距分析
第四章:竞争格局
- 介绍
- 公司矩阵分析
- 公司市占率分析
- 全球的
- 北美洲
- 欧洲
- 亚太地区
- 拉丁美洲
- 主要市场参与者的竞争分析
- 竞争定位矩阵
- 关键进展
- 併购
- 合作伙伴关係与合作
- 新产品发布
- 扩张计划
第五章:市场估算与预测:依测试类型划分,2022-2035年
- 类型 2
- 3型
- 4型
第六章:市场估算与预测:依应用领域划分,2022-2035年
- 阻塞性睡眠呼吸中止症(OSA)
- 中枢性睡眠呼吸中止症
第七章:市场估算与预测:依配销通路划分,2022-2035年
- 砖瓦
- 电子商务
第八章:市场估算与预测:依地区划分,2022-2035年
- 北美洲
- 我们
- 加拿大
- 欧洲
- 德国
- 英国
- 法国
- 西班牙
- 义大利
- 荷兰
- 亚太地区
- 中国
- 日本
- 印度
- 澳洲
- 韩国
- 拉丁美洲
- 巴西
- 墨西哥
- 阿根廷
- MEA
- 南非
- 沙乌地阿拉伯
- 阿联酋
第九章:公司简介
- CADWELL
- CleveMed
- COMPUMEDICS
- CONTEC
- natus
- Neurosoft
- NEUROVIRTUAL
- NIHON KOHDEN
- nox MEDICAL
- PHILIPS
- Resmed
- SOMNOmedics
- ZOLL itamar
The Global Home Sleep Apnea Testing Devices Market was valued at USD 1.6 billion in 2025 and is estimated to grow at a CAGR of 11.6% to reach USD 5 billion by 2035.

Market growth is fueled by the expanding elderly population, rising incidence of sleep apnea and related chronic conditions, and rapid innovation in portable and wearable diagnostic technologies. Increasing public awareness of sleep health and the clinical risks associated with undiagnosed sleep disorders is further supporting demand. Home-based testing solutions are gaining preference due to their affordability, convenience, and ability to deliver reliable diagnostic results outside traditional clinical environments. The growing adoption of telemedicine and remote patient monitoring is also accelerating uptake, as these devices integrate easily into digital healthcare workflows. The rising prevalence of obesity, diabetes, and cardiovascular disease is increasing the need for early screening and intervention, since untreated sleep apnea significantly elevates the risk of serious complications. Advances in sensor accuracy, wireless data transmission, and artificial intelligence are enabling devices that are smaller, more precise, and simpler for patients to operate independently. These factors are positioning home sleep apnea testing as a practical frontline diagnostic tool across global healthcare systems.
| Market Scope | |
|---|---|
| Start Year | 2025 |
| Forecast Year | 2026-2035 |
| Start Value | $1.6 Billion |
| Forecast Value | $5 Billion |
| CAGR | 11.6% |
The type 3 device segment held a 66.1% share in 2025. Growth in this segment is supported by increased emphasis on early diagnosis and preventive care. These devices track multiple physiological signals and offer a balance between clinical reliability and user convenience, making them suitable for identifying moderate to severe obstructive conditions in uncomplicated cases.
The obstructive sleep apnea segment was valued at USD 1.5 billion in 2025. This condition represents the most common form of sleep apnea and is closely associated with metabolic and cardiovascular risks, reinforcing the importance of timely diagnosis and monitoring.
North America Home Sleep Apnea Testing Devices Market accounted for a 33.6% share in 2025 and is expected to maintain strong growth. High awareness of sleep disorders, widespread telehealth adoption, advanced healthcare infrastructure, and elevated prevalence of lifestyle-related risk factors continue to drive regional demand.
Key companies active in the Global Home Sleep Apnea Testing Devices Market include ResMed, Philips, Nox Medical, Compumedics, ZOLL Itamar, Nihon Kohden, Natus, SOMNOmedics, CleveMed, Neurosoft, CADWELL, CONTEC, NEUROVIRTUAL, and Nox Medical. Companies in the Global Home Sleep Apnea Testing Devices Market are strengthening their market position through continuous product innovation, digital integration, and strategic partnerships. Manufacturers focus on improving diagnostic accuracy, comfort, and ease of use through advanced sensors and AI-driven analytics. Integration with telehealth platforms and cloud-based data management systems supports seamless clinical workflows. Many players invest in regulatory approvals and clinical validation to expand adoption across healthcare settings. Geographic expansion into underserved markets and collaboration with sleep clinics and healthcare providers help increase reach.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definition
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/Country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 360° synopsis
- 2.2 Key market trends
- 2.2.1 Regional trends
- 2.2.2 Test type trends
- 2.2.3 Indication trends
- 2.2.4 Distribution channel trends
- 2.3 CXO perspectives: Strategic imperatives
- 2.3.1 Key decision points for industry executives
- 2.3.2 Critical success factors for market players
- 2.4 Future outlook and strategic recommendations
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Growing prevalence of sleep apnea and related co-morbidities
- 3.2.1.2 Increasing awareness regarding sleep health and home sleep apnea tests
- 3.2.1.3 Rising aging population
- 3.2.1.4 Technological advancements in wearable and portable HSAT devices
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 Stringent regulatory approvals for diagnostic devices
- 3.2.2.2 Limited accuracy compared to in-lab polysomnography
- 3.2.3 Opportunities
- 3.2.3.1 Development of AI-powered diagnostic algorithms
- 3.2.3.2 Growth of telehealth platforms offering HSAT services
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.4.1 North America
- 3.4.2 Europe
- 3.4.3 Asia Pacific
- 3.4.4 LAMEA
- 3.5 Technology and innovation landscape
- 3.5.1 Current technological trends
- 3.5.2 Emerging technologies
- 3.6 Value chain analysis
- 3.7 Reimbursement scenario
- 3.8 Policy landscape
- 3.9 Consumer behavior insights
- 3.10 Porter's analysis
- 3.11 PESTEL analysis
- 3.12 Gap analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company matrix analysis
- 4.3 Company market share analysis
- 4.3.1 Global
- 4.3.2 North America
- 4.3.3 Europe
- 4.3.4 Asia Pacific
- 4.3.5 LAMEA
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Key developments
- 4.6.1 Mergers & acquisitions
- 4.6.2 Partnerships & collaborations
- 4.6.3 New product launches
- 4.6.4 Expansion plans
Chapter 5 Market Estimates and Forecast, By Test Type, 2022 - 2035 ($ Mn)
- 5.1 Key trends
- 5.2 Type 2
- 5.3 Type 3
- 5.4 Type 4
Chapter 6 Market Estimates and Forecast, By Application, 2022 - 2035 ($ Mn)
- 6.1 Key trends
- 6.2 Obstructive sleep apnea (OSA)
- 6.3 Central sleep apnea
Chapter 7 Market Estimates and Forecast, By Distribution Channel, 2022 - 2035 ($ Mn)
- 7.1 Key trends
- 7.2 Brick and mortar
- 7.3 E-commerce
Chapter 8 Market Estimates and Forecast, By Region, 2022 - 2035 ($ Mn)
- 8.1 Key trends
- 8.2 North America
- 8.2.1 U.S.
- 8.2.2 Canada
- 8.3 Europe
- 8.3.1 Germany
- 8.3.2 UK
- 8.3.3 France
- 8.3.4 Spain
- 8.3.5 Italy
- 8.3.6 Netherlands
- 8.4 Asia Pacific
- 8.4.1 China
- 8.4.2 Japan
- 8.4.3 India
- 8.4.4 Australia
- 8.4.5 South Korea
- 8.5 Latin America
- 8.5.1 Brazil
- 8.5.2 Mexico
- 8.5.3 Argentina
- 8.6 MEA
- 8.6.1 South Africa
- 8.6.2 Saudi Arabia
- 8.6.3 UAE
Chapter 9 Company Profiles
- 9.1 CADWELL
- 9.2 CleveMed
- 9.3 COMPUMEDICS
- 9.4 CONTEC
- 9.5 natus
- 9.6 Neurosoft
- 9.7 NEUROVIRTUAL
- 9.8 NIHON KOHDEN
- 9.9 nox MEDICAL
- 9.10 PHILIPS
- 9.11 Resmed
- 9.12 SOMNOmedics
- 9.13 ZOLL itamar











