 |
市场调查报告书
商品编码
1800731
全球 RWE(真实世界证据)解决方案市场(至 2030 年)按组件(资料集)、应用(药物/设备开发(肿瘤学、心臟病学、神经病学)、报销)、最终用户(药品、医疗技术、付款人、医疗保健提供者)和地区划分Real World Evidence Solutions Market by Component (Datasets), Application (Drug/Device Development (Cancer, Cardio, Neuro), Reimbursement), End User (Pharma, Medtech, Payers, Provider), Region - Global Forecast to 2030 |
||||||
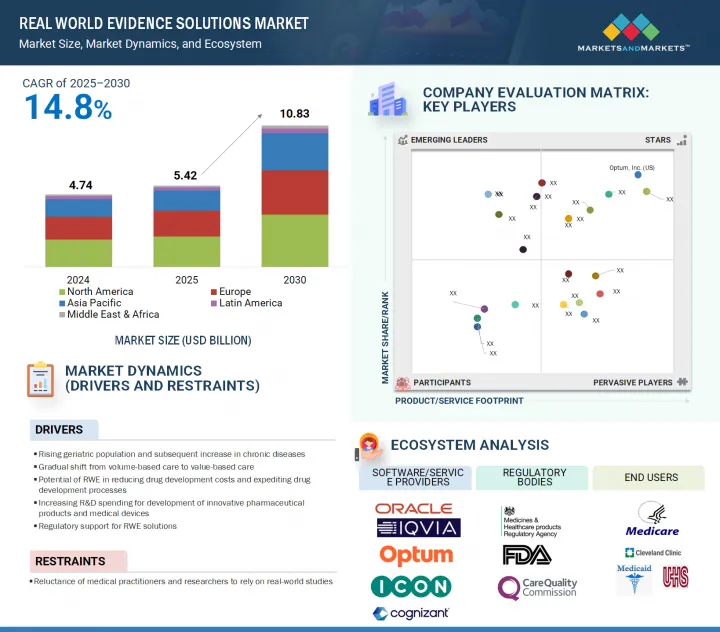
全球 RWE(真实世界证据)解决方案市场预计将从 2025 年的 54.2 亿美元成长到 2030 年的 108.3 亿美元,预测期内的复合年增长率为 14.8%。
| 调查范围 | |
|---|---|
| 调查年份 | 2024-2030 |
| 基准年 | 2024 |
| 预测期 | 2025-2030 |
| 单元 | 金额(美元) |
| 部分 | 组件、用途、部署模式、收益模式和最终用户 |
| 目标区域 | 北美、欧洲、亚太地区、拉丁美洲、中东和非洲 |
市场成长的驱动力源自于向价值导向型医疗保健的转变以及慢性病发病率的上升。真实世界证据 (RWE) 有望降低药物开发过程中的成本和时间,这使得製药和医疗设备公司越来越多地采用 RWE 解决方案。
“根据应用,预计上市后监测部门在预测期内将实现最高的复合年增长率。”
药品和医疗设备上市后的长期安全性和有效性日益受到监管重视,这推动了该领域的快速发展。美国FDA、欧洲EMA和日本PMDA等监管机构高度重视监测真实世界结果、识别不利事件、追踪产品性能以及支援风险管理规划。
“根据最终用户来看,製药和生物技术行业预计在预测期内以最高的复合年增长率增长。”
市场成长的主要驱动力是RWE解决方案的日益普及,因为RWE数据能够为新药和医疗设备的开发提供临床洞察,从而降低开发成本和时间。製药业本身的显着成长也是加速RWE解决方案普及的另一个因素。
预计亚太地区在预测期内的复合年增长率最高。
这种快速扩张的动力来自于中国、印度、日本和韩国等国家开展的大量临床试验,以及不断发展的法规结构开始在药品核准和上市后监测中采用 RWE。
本报告调查了全球 RWE(真实世界证据)解决方案市场,并提供了市场概况、影响市场成长的各种因素分析、技术和专利趋势、法律制度、案例研究、市场规模趋势和预测、各个细分市场、地区/主要国家的详细分析、竞争格局以及主要企业的概况。
目录
第一章 引言
第二章调查方法
第三章执行摘要
第四章重要考察
第五章市场概述
- 市场动态
- 驱动程式
- 抑制因素
- 机会
- 任务
- 产业趋势
- 穿戴式装置的新角色
- 社群媒体衍生的RWE
- 製药业中 RWD 和 RWE 的使用日益增多
- RWE 分析法:内部分析 vs. 外包分析
- 将人工智慧引入 RWD 管理
- 真实世界的资料来源
- 生态系分析
- 价值链分析
- 影响客户业务的趋势/中断
- 波特五力分析
- 定价分析
- 技术分析
- 关税和监管状况
- 专利分析
- 大型会议及活动
- 主要相关人员和采购标准
- 案例研究分析
- 最终用户分析
- RWE 解决方案市场:经营模式
- 投资金筹措场景
- 人工智慧/生成式人工智慧对RWE解决方案市场的影响
- 美国关税
6. RWE(真实世界证据)解决方案市场(按组件)
- 服务
- 数据集
- 异质资料集
- 集成资料集
7. 真实世界证据 (RWE) 解决方案市场(按应用)
- 药物研发与核准
- 瘤
- 心血管疾病
- 神经
- 免疫
- 罕见疾病
- 其他的
- 医疗设备开发与核准
- 上市后监测
- 市场进入和报销/覆盖决策
- 临床和监管决策
- 其他的
第 8 章:真实世界证据 (RWE) 解决方案市场(按收益模式)
- 计量收费(基于价值的定价)
- 订阅
- 执照
9. 真实世界证据 (RWE) 解决方案市场(按部署模式)
- 本地
- 云端基础
- 杂交种
第 10 章。 RWE(真实世界证据)解决方案市场(按最终用户)
- 製药和生物技术公司
- 医疗技术公司
- 保险公司
- 公共保险公司
- 私人保险公司
- 医疗保健提供者
- 医院
- 诊所及其他门诊部
- 其他的
- 其他的
11. RWE(真实世界证据)解决方案市场(按地区)
- 北美洲
- 宏观经济展望
- 美国
- 加拿大
- 欧洲
- 宏观经济展望
- 德国
- 英国
- 法国
- 义大利
- 西班牙
- 其他的
- 亚太地区
- 宏观经济展望
- 日本
- 中国
- 印度
- 澳洲
- 韩国
- 其他的
- 拉丁美洲
- 宏观经济展望
- 巴西
- 墨西哥
- 其他的
- 中东和非洲
- 宏观经济展望
- 海湾合作委员会国家
- 南非
- 其他的
第十二章竞争格局
- 主要参与企业的策略/优势
- 收益份额分析
- 市场占有率分析
- 公司评估矩阵:主要企业
- 公司评估矩阵:Start-Ups/中小企业
- 品牌/产品比较
- 估值和财务指标
- 竞争场景
第十三章:公司简介
- 主要企业
- IQVIA
- MERATIVE
- OPTUM, INC.
- ICON PLC
- SYNEOS HEALTH
- PAREXEL INTERNATIONAL (MA) CORPORATION
- FLATIRON HEALTH
- FORTREA
- ORACLE
- ELEVANCE HEALTH
- SAS INSTITUTE INC.
- AETION, INC.
- TRINETX, LLC
- TRINITY
- MEDIDATA
- COGNIZANT TECHNOLOGY SOLUTIONS CORPORATION
- CEGEDIM HEALTH DATA
- VERANTOS
- MEDPACE
- TATA CONSULTANCY SERVICES LIMITED
- 其他公司
- HEALTHVERITY, INC.
- OM1
- OPEN HEALTH
- TEMPUS
- QUANTZIG
第十四章 附录
The global real world evidence solutions market is projected to reach USD 10.83 billion by 2030 from USD 5.42 billion in 2025, at a CAGR of 14.8% during the forecast period.
| Scope of the Report | |
|---|---|
| Years Considered for the Study | 2024-2030 |
| Base Year | 2024 |
| Forecast Period | 2025-2030 |
| Units Considered | Value (USD billion) |
| Segments | Component, Application, Deployment Mode, Revenue Model, and End User |
| Regions covered | North America, Europe, the Asia Pacific, Latin America, and the Middle East & Africa |
The market's growth is fueled by the transition to value-based care and the increasing incidence of chronic diseases. Real world evidence (RWE) can potentially reduce both costs and time in the drug development process. This has resulted in the growing adoption of RWE solutions across pharmaceutical & medical device companies.

"By component, the data sets segment is expected to grow at the highest CAGR during the forecast period."
Based on component, the real world evidence solution market is segmented into services, data sets, and integrated data sets. The data sets segment is projected to grow at the highest CAGR during the forecast period. With the growing digitization of healthcare and increasing acceptance of real world data by regulators such as the FDA and EMA; the demand for high-quality, curated datasets, such as EHRs, claims, and registry data, has surged. These datasets power analytics, AI models, and health economics studies, making them critical assets for pharma, biotech, and healthcare companies. Additionally, their recurring revenue potential through licensing further drives their dominance in the RWE landscape.
"By application, the post-market surveillance segment is expected to register the highest CAGR during the forecast period."
By application, the real world evidence solutions market is segmented into drug development & approvals, medical device development & approvals, post-market surveillance, market access & reimbursement decision-making, clinical & regulatory decision-making, and other applications. The post-market surveillance segment is expected to register the highest CAGR during the forecast period. The increasing regulatory focus on long-term safety and the efficacy of drugs & medical devices after market approval drives the high growth rate of this segment. Regulatory agencies such as the US FDA, the EMA, and the PMDA in Japan have emphasized monitoring real-world outcomes to identify adverse events, track product performance, and support risk management plans.
"By end user, the pharmaceutical & biotechnology companies segment is projected to grow at the highest CAGR during the forecast period."
The RWE solutions market is segmented by end users into pharmaceutical & biotechnology companies, MedTech companies, healthcare payers, healthcare providers, and other end users. The pharmaceutical & biotechnological companies segment is projected to grow at the highest CAGR during the forecast period. The key factors contributing to market growth include adopting RWE solutions to decrease the costs & time for drug development, as RWE data provides clinical insights on the development of new drugs & medical devices. The high growth in the pharmaceutical industry is also an additional factor, fueling the adoption of real world solutions.
"The Asia Pacific region is projected to register the highest CAGR during the forecast period."
The market is segmented by region into North America, Europe, the Asia Pacific, Latin America, and the Middle East & Africa. The RWE solutions market in the Asia Pacific region is projected to register the highest CAGR during the forecast period. The high growth rate of this region is attributed to the rising initiatives towards adopting RWE by pharmaceutical & medical device companies. This rapid expansion is driven by the high volume of clinical trials undertaken by emerging economies such as China, India, Japan, and South Korea, along with evolving regulatory frameworks that are beginning to accept RWE for drug approvals and post-market surveillance.
Breakdown of supply-side primary interviews, by company type, designation, and region:
- By Company Type: Tier 1 (40%), Tier 2 (35%), and Tier 3 (25%)
- By Designation: C-level (35%), Director-level (45%), and Others (20%)
- By Region: North America (55%), Europe (20%), the Asia Pacific (15%), Latin America (5%), and the Middle East & Africa (5%)
List of Companies Profiled in the Report
- IQVIA Inc. (US)
- Merative (US)
- Optum Inc. (US)
- ICON Plc (Ireland)
- Syneos Health (US)
- Parexel International Corporation (US)
- Tata Consultancy Services (India)
- Oracle (US)
- Elevance Health (US)
- SAS Institute Inc. (US)
- Aetion Inc. (US)
- Trinetx LLC (US)
- Trinity (US)
- Cognizant Technology Solutions Corporation (US)
- Cegedim Health Data (France)
- Verantos (US)
- Medspace Holdings Inc. (US)
- FLATIRON HEALTH (US)
- Fortrea (US)
- Medidata (US) (subsidiary of Dassault Systemes)
- HealthVerity, Inc. (US)
- Quantzig (US)
- OPEN Health (UK)
- Tempus (US)
- Certara (US)
Research Coverage
This report studies the real world evidence solutions market based on component, application, revenue model, deployment mode, end user, and region. The report also analyzes market growth factors (drivers, restraints, opportunities, and challenges). It evaluates the market's opportunities and challenges for stakeholders and details the competitive landscape for market leaders. The report also studies micro markets concerning their growth trends, prospects, and contributions to the total real world evidence solutions market. The report forecasts the revenue of the market segments in five regions.
Reasons to Buy the Report
This report also includes.
- Analysis of key drivers (rising geriatric population and rise in incidences of chronic diseases, shift from volume-based care to value-based care, potential of RWE in reducing drug development costs and expediting drug development process, increased R&D spending for development of new pharmaceutical products and medical devices, and support from regulatory bodies for use of RWE solutions), restraints (reluctance of medical practitioners and researchers to rely on real-world studies), opportunities (increase focus on end-to-end RWE services, and growth opportunities in emerging markets), and challenges (scarcity of skilled personnel, and the lack of universally accepted methodology principles and data processing infrastructure).
- Product Development/Innovation: Detailed insights on upcoming trends, research & development activities, and new software launches in the real world evidence solutions market.
- Market Development: Comprehensive information on the lucrative emerging markets, by component, application, revenue model, deployment mode, end user, and region.
- Market Diversification: Exhaustive information about the software portfolios, growing geographies, recent developments, and investments in the real world evidence solutions market.
- Competitive Assessment: In-depth assessment of market shares, growth strategies, product offerings, company evaluation matrix, and capabilities of leading players in the global real world evidence solutions market.
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 STUDY OBJECTIVES
- 1.2 MARKET DEFINITION
- 1.3 STUDY SCOPE
- 1.3.1 MARKET SEGMENTATION
- 1.3.2 INCLUSIONS & EXCLUSIONS
- 1.4 YEARS CONSIDERED
- 1.5 CURRENCY CONSIDERED
- 1.6 STAKEHOLDERS
- 1.7 SUMMARY OF CHANGES
2 RESEARCH METHODOLOGY
- 2.1 RESEARCH DATA
- 2.1.1 SECONDARY DATA
- 2.1.1.1 Key data from secondary sources
- 2.1.2 PRIMARY DATA
- 2.1.2.1 Key data from primary sources
- 2.1.2.2 Key industry insights
- 2.1.1 SECONDARY DATA
- 2.2 RESEARCH METHODOLOGY DESIGN
- 2.3 MARKET SIZE ESTIMATION
- 2.3.1 BOTTOM-UP APPROACH
- 2.3.2 TOP-DOWN APPROACH
- 2.4 MARKET BREAKDOWN AND DATA TRIANGULATION
- 2.5 RESEARCH ASSUMPTIONS
- 2.6 RISK ASSESSMENT
- 2.7 LIMITATIONS
- 2.7.1 SCOPE-RELATED LIMITATIONS
- 2.7.2 METHODOLOGY-RELATED LIMITATIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
- 4.1 REAL WORLD EVIDENCE SOLUTIONS MARKET OVERVIEW
- 4.2 ASIA PACIFIC: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY END USER AND COUNTRY
- 4.3 REAL WORLD EVIDENCE SOLUTIONS MARKET: GEOGRAPHIC GROWTH OPPORTUNITIES
- 4.4 REGIONAL MIX: REAL WORLD EVIDENCE SOLUTIONS MARKET, 2025-2030
- 4.5 REAL WORLD EVIDENCE SOLUTIONS MARKET: DEVELOPED MARKETS VS. EMERGING ECONOMIES, 2025 VS. 2030
5 MARKET OVERVIEW
- 5.1 INTRODUCTION
- 5.2 MARKET DYNAMICS
- 5.2.1 DRIVERS
- 5.2.1.1 Rising geriatric population and subsequent increase in prevalence of chronic diseases
- 5.2.1.2 Shift from volume-based care to value-based care
- 5.2.1.3 Potential of real world evidence in reducing drug development costs and expediting drug development process
- 5.2.1.4 Increased R&D spending for development of new pharmaceutical products and medical devices
- 5.2.1.5 Support from regulatory bodies for use of real world evidence solutions
- 5.2.2 RESTRAINTS
- 5.2.2.1 Reluctance of medical practitioners and researchers to rely on real world studies
- 5.2.2.2 Data quality and standardization issues
- 5.2.3 OPPORTUNITIES
- 5.2.3.1 Growth opportunities in emerging markets
- 5.2.3.2 Increased focus on end-to-end RWE services
- 5.2.3.3 Increased focus on personalized and precision medicine
- 5.2.3.4 Market access, health technology assessment (HTA), and reimbursement
- 5.2.4 CHALLENGES
- 5.2.4.1 Lack of universally accepted methodology standards and data processing infrastructure
- 5.2.4.2 Shortage of skilled professionals
- 5.2.1 DRIVERS
- 5.3 INDUSTRY TRENDS
- 5.3.1 EMERGING ROLE OF WEARABLE DEVICES
- 5.3.2 SOCIAL MEDIA-SOURCED RWE
- 5.3.3 RISING USE OF RWD AND RWE ACROSS PHARMACEUTICAL INDUSTRY
- 5.3.4 RWE ANALYTICS APPROACH: INTERNAL VS. OUTSOURCED
- 5.3.5 INCORPORATION OF ARTIFICIAL INTELLIGENCE INTO RWD MANAGEMENT
- 5.4 REAL WORLD DATA SOURCES
- 5.5 ECOSYSTEM ANALYSIS
- 5.6 VALUE CHAIN ANALYSIS
- 5.7 TRENDS/DISRUPTIONS IMPACTING CUSTOMER BUSINESS
- 5.8 PORTER'S FIVE FORCES ANALYSIS
- 5.8.1 THREAT FROM NEW ENTRANTS
- 5.8.2 BARGAINING POWER OF SUPPLIERS
- 5.8.3 BARGAINING POWER OF BUYERS
- 5.8.4 THREAT FROM SUBSTITUTES
- 5.8.5 INTENSITY OF COMPETITIVE RIVALRY
- 5.9 PRICING ANALYSIS
- 5.9.1 INDICATIVE PRICING FOR REAL WORLD EVIDENCE SOLUTIONS, BY COMPONENT
- 5.9.2 INDICATIVE PRICING OF REAL WORLD EVIDENCE SOLUTIONS, BY REGION
- 5.10 TECHNOLOGY ANALYSIS
- 5.10.1 KEY TECHNOLOGIES
- 5.10.1.1 Use of AI and ML
- 5.10.1.2 Blockchain technology
- 5.10.2 ADJACENT TECHNOLOGIES
- 5.10.2.1 Predictive analytics
- 5.10.2.2 Visualization dashboard software
- 5.10.3 COMPLEMENTARY TECHNOLOGIES
- 5.10.3.1 Electronic health records (EHRs)
- 5.10.3.2 Clinical trial management system
- 5.10.1 KEY TECHNOLOGIES
- 5.11 TARIFF AND REGULATORY LANDSCAPE
- 5.11.1 REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- 5.11.2 REGULATORY ANALYSIS
- 5.11.2.1 North America
- 5.11.2.1.1 US
- 5.11.2.1.1.1 Use cases
- 5.11.2.1.2 Canada
- 5.11.2.1.1 US
- 5.11.2.2 Europe
- 5.11.2.3 Asia Pacific
- 5.11.2.3.1 China
- 5.11.2.3.2 Japan
- 5.11.2.3.3 India
- 5.11.2.4 Middle East & Africa
- 5.11.2.5 Latin America
- 5.11.2.1 North America
- 5.12 PATENT ANALYSIS
- 5.12.1 PATENT PUBLICATION TRENDS FOR REAL WORLD EVIDENCE SOLUTIONS MARKET
- 5.12.2 JURISDICTION AND TOP APPLICANT ANALYSIS
- 5.13 KEY CONFERENCES & EVENTS
- 5.14 KEY STAKEHOLDERS & BUYING CRITERIA
- 5.14.1 KEY STAKEHOLDERS IN BUYING PROCESS
- 5.14.2 BUYING CRITERIA
- 5.15 CASE STUDY ANALYSIS
- 5.15.1 DESIGNING AND BUILDING RWE DASHBOARDS AND LEVERAGING EHR AND CLAIMS DATA
- 5.15.2 INTEGRATING SPECIALTY PHARMACY AND PATIENT HUB DATA WITH RWD DATA TO INVESTIGATE INTO NON-ADHERENCE FACTORS
- 5.15.3 SIMPLIFYING DATA ANALYTICS WITH RWE PLATFORM
- 5.16 END USER ANALYSIS
- 5.16.1 UNMET NEEDS
- 5.16.2 END USER EXPECTATIONS
- 5.17 REAL WORLD EVIDENCE SOLUTIONS MARKET: BUSINESS MODELS
- 5.17.1 PLATFORM-AS-A-SERVICE (PAAS) MODEL
- 5.17.2 DATA PROVIDER MODEL
- 5.17.3 CONSULTING AND SERVICES MODEL
- 5.17.4 COLLABORATIVE RESEARCH MODEL
- 5.18 INVESTMENT & FUNDING SCENARIO
- 5.19 IMPACT OF AI/GENERATIVE AI ON REAL WORLD EVIDENCE SOLUTIONS MARKET
- 5.19.1 TOP USE CASES & MARKET POTENTIAL
- 5.19.1.1 Key use cases
- 5.19.2 IMPLEMENTATION OF AI/GENERATIVE AI: CASE STUDIES
- 5.19.2.1 AI-powered RWE transformed patient targeting and treatment initiation
- 5.19.3 IMPACT OF GENERATIVE AI ON INTERCONNECTED AND ADJACENT MARKET ECOSYSTEM
- 5.19.3.1 Electronic health record (EHR) market
- 5.19.3.2 Real world data (RWD) market
- 5.19.4 USER READINESS AND IMPACT ASSESSMENT
- 5.19.1 TOP USE CASES & MARKET POTENTIAL
- 5.20 US TARIFF 2025
- 5.20.1 INTRODUCTION
- 5.20.2 KEY TARIFF RATES
- 5.20.3 PRICE IMPACT ANALYSIS
- 5.20.4 IMPACT ON COUNTRY/REGION
- 5.20.4.1 US
- 5.20.4.2 Europe
- 5.20.4.3 Asia Pacific
- 5.20.5 IMPACT ON END-USE INDUSTRIES
6 REAL WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT
- 6.1 INTRODUCTION
- 6.2 SERVICES
- 6.2.1 RISING NEED TO CONVERT DATA INTO ACTIONABLE EVIDENCE TO DRIVE DEMAND
- 6.3 DATA SETS
- 6.3.1 DISPARATE DATA SETS
- 6.3.1.1 Clinical settings data sets
- 6.3.1.1.1 Increasing utilization of EHR data for trial recruitment to fuel growth
- 6.3.1.2 Claims data sets
- 6.3.1.2.1 Growing need to understand economic benefits of drug reimbursement by payers to drive growth
- 6.3.1.3 Pharmacy data sets
- 6.3.1.3.1 Increasing adoption of e-prescribing systems to drive growth
- 6.3.1.4 Patient-powered data sets
- 6.3.1.4.1 Increasing need to access opinions on diseases and treatments across social media to drive growth
- 6.3.1.5 Registry-based data sets
- 6.3.1.5.1 Increasing number of disease registries to drive demand for registry-based data sets in evidence generation
- 6.3.1.6 Others
- 6.3.1.6.1 Increasing number of genomics registries to drive demand for RWE evidence solutions
- 6.3.1.1 Clinical settings data sets
- 6.3.2 INTEGRATED DATA SETS
- 6.3.2.1 Increasing demand for integrated data from multiple sources to drive growth
- 6.3.1 DISPARATE DATA SETS
7 REAL WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION
- 7.1 INTRODUCTION
- 7.2 DRUG DEVELOPMENT & APPROVALS
- 7.2.1 ONCOLOGY
- 7.2.1.1 Growing number of clinical trials focused on cancer treatment to drive demand
- 7.2.2 CARDIOVASCULAR DISEASES
- 7.2.2.1 High prevalence of cardiovascular diseases to support market growth
- 7.2.3 NEUROLOGY
- 7.2.3.1 Rapidly aging global population and subsequent increase in prevalence of neurological disorders to drive growth
- 7.2.4 IMMUNOLOGY
- 7.2.4.1 Increasing focus on developing innovative products to drive growth
- 7.2.5 RARE DISEASES
- 7.2.5.1 Need for harnessing RWE to advance therapies for rare diseases to boost market
- 7.2.6 OTHER THERAPEUTIC AREAS
- 7.2.1 ONCOLOGY
- 7.3 MEDICAL DEVICE DEVELOPMENT & APPROVALS
- 7.3.1 INCREASING RESEARCH IN MEDICAL DEVICE DEVELOPMENT TO DRIVE DEMAND
- 7.4 POST-MARKET SURVEILLANCE
- 7.4.1 EXTENSIVE USE OF RWE SOLUTIONS IN POST-MARKET SURVEILLANCE TO BOOST MARKET
- 7.5 MARKET ACCESS & REIMBURSEMENT/COVERAGE DECISION-MAKING
- 7.5.1 GROWING USE OF RWE SOLUTIONS TO DEVELOP ECONOMIC AND BUDGET IMPACT MODELS TO FAVOR MARKET GROWTH
- 7.6 CLINICAL & REGULATORY DECISION-MAKING
- 7.6.1 LIMITED VALIDITY OF RANDOMIZED CONTROLLED TRIALS TO DRIVE USE OF RWE SOLUTIONS
- 7.7 OTHER APPLICATIONS
8 REAL WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL
- 8.1 INTRODUCTION
- 8.2 PAY-PER-USAGE (VALUE-BASED PRICING)
- 8.2.1 RISING DEMAND FOR COST-EFFECTIVE AND FLEXIBLE SOLUTIONS TO BOOST MARKET
- 8.3 SUBSCRIPTION
- 8.3.1 RISING PREFERENCE FOR FLEXIBILITY AND SCALABILITY TO SUPPORT MARKET GROWTH
- 8.4 LICENSE
- 8.4.1 FOCUS ON ENABLING END-TO-END RWE DELIVERY THROUGH PLATFORM LICENSING TO DRIVE MARKET
9 REAL WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE
- 9.1 INTRODUCTION
- 9.2 ON-PREMISES
- 9.2.1 NEED FOR ENHANCED DATA CONTROL BENEFITS TO BOOST DEMAND
- 9.3 CLOUD-BASED
- 9.3.1 INCREASED SCALABILITY AND FLEXIBILITY IN DATA COLLECTION TO DRIVE GROWTH
- 9.4 HYBRID
- 9.4.1 EMPHASIS ON INCREASED SCALABILITY AND FLEXIBILITY IN DATA COLLECTION TO DRIVE GROWTH
10 REAL WORLD EVIDENCE SOLUTIONS MARKET, BY END USER
- 10.1 INTRODUCTION
- 10.2 PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES
- 10.2.1 INCREASED R&D EXPENDITURE IN INNOVATIVE MEDICINES TO DRIVE MARKET GROWTH
- 10.3 MEDTECH COMPANIES
- 10.3.1 NEED FOR ACCELERATING MEDTECH MARKET ACCESS VIA REAL WORLD EVIDENCE SOLUTIONS TO BOOST GROWTH
- 10.4 HEALTHCARE PAYERS
- 10.4.1 INCREASED FOCUS ON OUTCOME-BASED PAYMENT MODELS TO DRIVE DEMAND
- 10.5 PUBLIC PAYERS
- 10.5.1 FOCUS ON ENHANCING PUBLIC PAYER DECISION-MAKING WITH RWE SOLUTIONS TO BOOST MARKET
- 10.6 PRIVATE PAYERS
- 10.6.1 NEED FOR LEVERAGING PRIVATE PAYERS FOR SUSTAINABLE RWE SOLUTIONS TO SPUR DEMAND
- 10.7 HEALTHCARE PROVIDERS
- 10.7.1 GROWING FOCUS ON IMPROVING PROFITABILITY TO SUPPORT MARKET GROWTH
- 10.7.2 HOSPITALS
- 10.7.3 CLINICS & OTHER OUTPATIENT SETTINGS
- 10.7.4 OTHER HEALTHCARE PROVIDERS
- 10.8 OTHER END USERS
11 REAL WORLD EVIDENCE SOLUTIONS MARKET, BY REGION
- 11.1 INTRODUCTION
- 11.2 NORTH AMERICA
- 11.2.1 NORTH AMERICA: MACROECONOMIC OUTLOOK
- 11.2.2 US
- 11.2.2.1 US to account for significant share of North American market
- 11.2.3 CANADA
- 11.2.3.1 Increase in pharma hub growth in Canada to drive market
- 11.3 EUROPE
- 11.3.1 EUROPE: MACROECONOMIC OUTLOOK
- 11.3.2 GERMANY
- 11.3.2.1 High pharmaceutical R&D spending in Germany to boost market growth
- 11.3.3 UK
- 11.3.3.1 Growing adoption of HTA to support market growth
- 11.3.4 FRANCE
- 11.3.4.1 Need for extensive and diversified database to drive market growth
- 11.3.5 ITALY
- 11.3.5.1 High demand for RWE due to widespread use of pay-for-outcomes to drive market
- 11.3.6 SPAIN
- 11.3.6.1 Rising R&D expenditure to propel market growth
- 11.3.7 REST OF EUROPE
- 11.4 ASIA PACIFIC
- 11.4.1 ASIA PACIFIC: MACROECONOMIC OUTLOOK
- 11.4.2 JAPAN
- 11.4.2.1 Stringent regulatory scenario in Japan to restrain market growth
- 11.4.3 CHINA
- 11.4.3.1 Low cost of clinical trials and large pharmaceutical R&D base in China to drive market
- 11.4.4 INDIA
- 11.4.4.1 Growing adoption of outcome-based research to drive market
- 11.4.5 AUSTRALIA
- 11.4.5.1 Policy reforms and strong regulations to support market growth
- 11.4.6 SOUTH KOREA
- 11.4.6.1 Increased focus on pharma R&D to boost market growth
- 11.4.7 REST OF ASIA PACIFIC
- 11.5 LATIN AMERICA
- 11.5.1 LATIN AMERICA: MACROECONOMIC OUTLOOK
- 11.5.2 BRAZIL
- 11.5.2.1 Evolving regulatory frameworks that facilitate clinical research to drive market
- 11.5.3 MEXICO
- 11.5.3.1 Increased funding and investments in pharma R&D to favor growth
- 11.5.4 REST OF LATIN AMERICA
- 11.6 MIDDLE EAST & AFRICA
- 11.6.1 MIDDLE EAST & AFRICA: MACROECONOMIC OUTLOOK
- 11.6.2 GCC COUNTRIES
- 11.6.2.1 Growing availability of healthcare funding to offer opportunities for market growth
- 11.6.2.2 Saudi Arabia
- 11.6.2.2.1 Digital health reform and regulatory innovation to offer opportunities for market growth
- 11.6.2.3 UAE
- 11.6.2.3.1 Need for driving data-driven healthcare transformation to boost market
- 11.6.2.4 Rest of GCC
- 11.6.3 SOUTH AFRICA
- 11.6.3.1 Emphasis on supporting healthcare infrastructure to drive market growth
- 11.6.4 REST OF MIDDLE EAST & AFRICA
12 COMPETITIVE LANDSCAPE
- 12.1 INTRODUCTION
- 12.2 KEY PLAYER STRATEGIES/RIGHT TO WIN
- 12.2.1 OVERVIEW OF STRATEGIES ADOPTED BY KEY PLAYERS
- 12.3 REVENUE SHARE ANALYSIS
- 12.4 MARKET SHARE ANALYSIS
- 12.5 COMPANY EVALUATION MATRIX: KEY PLAYERS
- 12.5.1 STARS
- 12.5.2 EMERGING LEADERS
- 12.5.3 PERVASIVE PLAYERS
- 12.5.4 PARTICIPANTS
- 12.5.5 COMPANY FOOTPRINT
- 12.6 COMPANY EVALUATION MATRIX: STARTUPS/SMES
- 12.6.1 PROGRESSIVE COMPANIES
- 12.6.2 RESPONSIVE COMPANIES
- 12.6.3 DYNAMIC COMPANIES
- 12.6.4 STARTING BLOCKS
- 12.6.5 COMPETITIVE BENCHMARKING
- 12.7 BRAND/PRODUCT COMPARISON
- 12.8 VALUATION AND FINANCIAL METRICS
- 12.9 COMPETITIVE SCENARIO
- 12.9.1 PRODUCT LAUNCHES/ENHANCEMENTS
- 12.9.2 DEALS
- 12.9.3 OTHER DEVELOPMENTS
13 COMPANY PROFILES
- 13.1 KEY PLAYERS
- 13.1.1 IQVIA
- 13.1.1.1 Business overview
- 13.1.1.2 Products offered
- 13.1.1.3 Recent developments
- 13.1.1.3.1 Deals
- 13.1.1.4 MnM view
- 13.1.1.4.1 Key strengths/Right to win
- 13.1.1.4.2 Strategic choices
- 13.1.1.4.3 Weaknesses and competitive threats
- 13.1.2 MERATIVE
- 13.1.2.1 Business overview
- 13.1.2.2 Products offered
- 13.1.2.3 Recent developments
- 13.1.2.3.1 Deals
- 13.1.2.3.2 Other developments
- 13.1.2.4 MnM view
- 13.1.2.4.1 Key strengths/Right to win
- 13.1.2.4.2 Strategic choices
- 13.1.2.4.3 Weaknesses and competitive threats
- 13.1.3 OPTUM, INC.
- 13.1.3.1 Business overview
- 13.1.3.2 Products offered
- 13.1.3.3 Recent developments
- 13.1.3.3.1 Product launches/developments
- 13.1.3.3.2 Deals
- 13.1.3.4 MnM view
- 13.1.3.4.1 Key strengths/Right to win
- 13.1.3.4.2 Strategic choices made
- 13.1.3.4.3 Weaknesses and competitive threats
- 13.1.4 ICON PLC
- 13.1.4.1 Business overview
- 13.1.4.2 Products offered
- 13.1.4.3 Recent developments
- 13.1.4.3.1 Product launches/developments
- 13.1.4.3.2 Deals
- 13.1.4.4 MnM view
- 13.1.4.4.1 Key strengths/Right to win
- 13.1.4.4.2 Strategic choices
- 13.1.4.4.3 Weaknesses and competitive threats
- 13.1.5 SYNEOS HEALTH
- 13.1.5.1 Business overview
- 13.1.5.2 Products offered
- 13.1.5.3 Recent developments
- 13.1.5.3.1 Deals
- 13.1.5.4 MnM view
- 13.1.5.4.1 Key strengths/Right to win
- 13.1.5.4.2 Strategic choices
- 13.1.5.4.3 Weaknesses and competitive threats
- 13.1.6 PAREXEL INTERNATIONAL (MA) CORPORATION
- 13.1.6.1 Business overview
- 13.1.6.2 Products offered
- 13.1.6.3 Recent developments
- 13.1.6.3.1 Product launches/developments
- 13.1.6.3.2 Deals
- 13.1.7 FLATIRON HEALTH
- 13.1.7.1 Business overview
- 13.1.7.2 Products offered
- 13.1.7.3 Recent developments
- 13.1.7.3.1 Product launches/developments
- 13.1.7.3.2 Deals
- 13.1.8 FORTREA
- 13.1.8.1 Business overview
- 13.1.8.2 Products offered
- 13.1.8.3 Recent developments
- 13.1.8.3.1 Product launches/developments
- 13.1.8.3.2 Deals
- 13.1.9 ORACLE
- 13.1.9.1 Business overview
- 13.1.9.2 Products offered
- 13.1.9.3 Recent developments
- 13.1.9.3.1 Product launches/developments
- 13.1.9.3.2 Deals
- 13.1.10 ELEVANCE HEALTH
- 13.1.10.1 Business overview
- 13.1.10.2 Products offered
- 13.1.11 SAS INSTITUTE INC.
- 13.1.11.1 Business overview
- 13.1.11.2 Products offered
- 13.1.11.3 Recent developments
- 13.1.11.3.1 Product launches/developments
- 13.1.12 AETION, INC.
- 13.1.12.1 Business overview
- 13.1.12.2 Products offered
- 13.1.12.3 Recent developments
- 13.1.12.3.1 Product launches/developments
- 13.1.12.3.2 Deals
- 13.1.13 TRINETX, LLC
- 13.1.13.1 Business overview
- 13.1.13.2 Products offered
- 13.1.13.3 Recent developments
- 13.1.13.3.1 Product launches/developments
- 13.1.13.3.2 Deals
- 13.1.14 TRINITY
- 13.1.14.1 Business overview
- 13.1.14.2 Products offered
- 13.1.14.3 Recent developments
- 13.1.14.3.1 Deals
- 13.1.15 MEDIDATA
- 13.1.15.1 Business overview
- 13.1.15.2 Products offered
- 13.1.15.3 Recent developments
- 13.1.15.3.1 Product launches/developments
- 13.1.15.3.2 Deals
- 13.1.16 COGNIZANT TECHNOLOGY SOLUTIONS CORPORATION
- 13.1.16.1 Business overview
- 13.1.16.2 Products offered
- 13.1.17 CEGEDIM HEALTH DATA
- 13.1.17.1 Business overview
- 13.1.17.2 Products offered
- 13.1.17.3 Recent developments
- 13.1.17.3.1 Product launches/developments
- 13.1.17.3.2 Deals
- 13.1.18 VERANTOS
- 13.1.18.1 Business overview
- 13.1.18.2 Products offered
- 13.1.18.3 Recent developments
- 13.1.18.3.1 Product launches/developments
- 13.1.18.3.2 Deals
- 13.1.19 MEDPACE
- 13.1.19.1 Business overview
- 13.1.19.2 Products offered
- 13.1.20 TATA CONSULTANCY SERVICES LIMITED
- 13.1.20.1 Business overview
- 13.1.20.2 Products offered
- 13.1.20.3 Recent developments
- 13.1.20.3.1 Deals
- 13.1.1 IQVIA
- 13.2 OTHER PLAYERS
- 13.2.1 HEALTHVERITY, INC.
- 13.2.2 OM1
- 13.2.3 OPEN HEALTH
- 13.2.4 TEMPUS
- 13.2.5 QUANTZIG
14 APPENDIX
- 14.1 DISCUSSION GUIDE
- 14.2 KNOWLEDGESTORE: MARKETSANDMARKETS' SUBSCRIPTION PORTAL
- 14.3 CUSTOMIZATION OPTIONS
- 14.4 RELATED REPORTS
- 14.5 AUTHOR DETAILS
List of Tables
- TABLE 1 RISK ASSESSMENT ANALYSIS
- TABLE 2 MARKET DYNAMICS: IMPACT ANALYSIS
- TABLE 3 INDICATIVE LIST OF NATIONAL DATABASES IN DEVELOPED COUNTRIES
- TABLE 4 REAL WORLD EVIDENCE SOLUTIONS MARKET: ROLE OF PLAYERS IN ECOSYSTEM
- TABLE 5 PORTER'S FIVE FORCES ANALYSIS
- TABLE 6 INDICATIVE PRICING ANALYSIS OF REAL WORLD EVIDENCE SOLUTIONS, BY DATA SET, 2024 (USD)
- TABLE 7 INDICATIVE PRICE RANGE OF REAL WORLD EVIDENCE SOLUTIONS, BY KEY REGION, 2024 (USD)
- TABLE 8 REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 9 JURISDICTION ANALYSIS OF TOP APPLICANTS
- TABLE 10 LIST OF PATENTS, 2017-2025
- TABLE 11 DETAILED LIST OF CONFERENCES AND EVENTS
- TABLE 12 INFLUENCE OF STAKEHOLDERS ON BUYING PROCESS FOR KEY APPLICATIONS
- TABLE 13 KEY BUYING CRITERIA FOR KEY APPLICATIONS
- TABLE 14 UNMET NEEDS IN REAL WORLD EVIDENCE SOLUTIONS MARKET
- TABLE 15 END USER EXPECTATIONS IN REAL WORLD EVIDENCE SOLUTIONS MARKET
- TABLE 16 RECIPROCAL TARIFF RATES ADJUSTED BY US
- TABLE 17 REAL WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2023-2030 (USD MILLION)
- TABLE 18 REAL WORLD EVIDENCE SERVICES OFFERED BY KEY MARKET PLAYERS
- TABLE 19 REAL WORLD EVIDENCE SERVICES MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 20 REAL WORLD EVIDENCE DATA SETS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 21 REAL WORLD EVIDENCE DATA SETS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 22 REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 23 REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 24 CLINICAL SETTINGS DATA SETS OFFERED BY KEY MARKET PLAYERS
- TABLE 25 REAL WORLD EVIDENCE SOLUTIONS MARKET FOR CLINICAL SETTINGS DATA SETS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 26 CLAIMS DATA SETS OFFERED BY KEY MARKET PLAYERS
- TABLE 27 REAL WORLD EVIDENCE SOLUTIONS MARKET FOR CLAIMS DATA SETS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 28 REAL WORLD EVIDENCE SOLUTIONS MARKET FOR PHARMACY DATA SETS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 29 PATIENT-POWERED DATA SETS OFFERED BY KEY MARKET PLAYERS
- TABLE 30 REAL WORLD EVIDENCE SOLUTIONS MARKET FOR PATIENT-POWERED DATA SETS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 31 REAL WORLD EVIDENCE SOLUTIONS MARKET FOR REGISTRY-BASED DATA SETS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 32 REAL WORLD EVIDENCE SOLUTIONS MARKET FOR OTHER DATA SETS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 33 INTEGRATED DATA SETS OFFERED BY KEY MARKET PLAYERS
- TABLE 34 REAL WORLD EVIDENCE SOLUTIONS MARKET FOR INTEGRATED DATA SETS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 35 REAL WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 36 REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2023-2030 (USD MILLION)
- TABLE 37 REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 38 REAL WORLD EVIDENCE SOLUTIONS MARKET FOR ONCOLOGY, BY REGION, 2023-2030 (USD MILLION)
- TABLE 39 REAL WORLD EVIDENCE SOLUTIONS MARKET FOR CARDIOVASCULAR DISEASES, BY REGION, 2023-2030 (USD MILLION)
- TABLE 40 REAL WORLD EVIDENCE SOLUTIONS MARKET FOR NEUROLOGY, BY REGION, 2023-2030 (USD MILLION)
- TABLE 41 REAL WORLD EVIDENCE SOLUTIONS MARKET FOR IMMUNOLOGY, BY REGION, 2023-2030 (USD MILLION)
- TABLE 42 REAL WORLD EVIDENCE SOLUTIONS MARKET FOR RARE DISEASES, BY REGION, 2023-2030 (USD MILLION)
- TABLE 43 REAL WORLD EVIDENCE SOLUTIONS MARKET FOR OTHER THERAPEUTIC AREAS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 44 REAL WORLD EVIDENCE SOLUTIONS MARKET FOR MEDICAL DEVICE DEVELOPMENT & APPROVALS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 45 REAL WORLD EVIDENCE SOLUTIONS MARKET FOR POST-MARKET SURVEILLANCE, BY REGION, 2023-2030 (USD MILLION)
- TABLE 46 REAL WORLD EVIDENCE SOLUTIONS MARKET FOR MARKET ACCESS & REIMBURSEMENT/COVERAGE DECISION-MAKING, BY REGION, 2023-2030 (USD MILLION)
- TABLE 47 REAL WORLD EVIDENCE SOLUTIONS MARKET FOR CLINICAL & REGULATORY DECISION-MAKING, BY REGION, 2023-2030 (USD MILLION)
- TABLE 48 REAL WORLD EVIDENCE SOLUTIONS MARKET FOR OTHER APPLICATIONS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 49 REAL WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2023-2030 (USD MILLION)
- TABLE 50 REAL WORLD EVIDENCE SOLUTIONS MARKET FOR PAY-PER-USAGE (VALUE-BASED PRICING) MODEL, BY REGION, 2023-2030 (USD MILLION)
- TABLE 51 REAL WORLD EVIDENCE SOLUTIONS MARKET FOR SUBSCRIPTION MODEL, BY REGION, 2023-2030 (USD MILLION)
- TABLE 52 REAL WORLD EVIDENCE SOLUTIONS MARKET FOR LICENSE MODEL, BY REGION, 2023-2030 (USD MILLION)
- TABLE 53 REAL WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2023-2030 (USD MILLION)
- TABLE 54 REAL WORLD EVIDENCE SOLUTIONS MARKET FOR ON-PREMISES MODE, BY REGION, 2023-2030 (USD MILLION)
- TABLE 55 REAL WORLD EVIDENCE SOLUTIONS MARKET FOR CLOUD-BASED MODE, BY REGION, 2023-2030 (USD MILLION)
- TABLE 56 REAL WORLD EVIDENCE SOLUTIONS MARKET FOR HYBRID MODE, BY REGION, 2023-2030 (USD MILLION)
- TABLE 57 REAL WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 58 REAL WORLD EVIDENCE SOLUTIONS MARKET FOR PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES, BY REGION, 2023-2030 (USD MILLION)
- TABLE 59 REAL WORLD EVIDENCE SOLUTIONS MARKET FOR MEDTECH COMPANIES, BY REGION, 2023-2030 (USD MILLION)
- TABLE 60 REAL WORLD EVIDENCE SOLUTIONS MARKET FOR HEALTHCARE PAYERS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 61 REAL WORLD EVIDENCE SOLUTIONS MARKET FOR HEALTHCARE PAYERS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 62 REAL WORLD EVIDENCE SOLUTIONS MARKET FOR PUBLIC PAYERS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 63 REAL WORLD EVIDENCE SOLUTIONS MARKET FOR PRIVATE PAYERS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 64 REAL WORLD EVIDENCE SOLUTIONS MARKET FOR HEALTHCARE PROVIDERS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 65 REAL WORLD EVIDENCE SOLUTIONS MARKET FOR HEALTHCARE PROVIDERS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 66 REAL WORLD EVIDENCE SOLUTIONS MARKET FOR HOSPITALS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 67 REAL WORLD EVIDENCE SOLUTIONS MARKET FOR CLINICS & OTHER OUTPATIENT SETTINGS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 68 REAL WORLD EVIDENCE SOLUTIONS MARKET FOR OTHER HEALTHCARE PROVIDERS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 69 REAL WORLD EVIDENCE SOLUTIONS MARKET FOR OTHER END USERS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 70 REAL WORLD EVIDENCE SOLUTIONS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 71 NORTH AMERICA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 72 NORTH AMERICA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2023-2030 (USD MILLION)
- TABLE 73 NORTH AMERICA: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DATA SETS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 74 NORTH AMERICA: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 75 NORTH AMERICA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 76 NORTH AMERICA: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 77 NORTH AMERICA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2023-2030 (USD MILLION)
- TABLE 78 NORTH AMERICA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2023-2030 (USD MILLION)
- TABLE 79 NORTH AMERICA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 80 NORTH AMERICA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY HEALTHCARE PAYER, 2023-2030 (USD MILLION)
- TABLE 81 NORTH AMERICA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY HEALTHCARE PROVIDER, 2023-2030 (USD MILLION)
- TABLE 82 US: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2023-2030 (USD MILLION)
- TABLE 83 US: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DATA SETS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 84 US: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 85 US: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 86 US: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 87 US: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2023-2030 (USD MILLION)
- TABLE 88 US: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2023-2030 (USD MILLION)
- TABLE 89 US: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 90 US: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY HEALTHCARE PAYER, 2023-2030 (USD MILLION)
- TABLE 91 US: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY HEALTHCARE PROVIDER, 2023-2030 (USD MILLION)
- TABLE 92 CANADA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2023-2030 (USD MILLION)
- TABLE 93 CANADA: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DATA SETS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 94 CANADA: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 95 CANADA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 96 CANADA: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 97 CANADA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2023-2030 (USD MILLION)
- TABLE 98 CANADA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2023-2030 (USD MILLION)
- TABLE 99 CANADA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 100 CANADA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY HEALTHCARE PAYER, 2023-2030 (USD MILLION)
- TABLE 101 CANADA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY HEALTHCARE PROVIDER, 2023-2030 (USD MILLION)
- TABLE 102 EUROPE: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 103 EUROPE: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2023-2030 (USD MILLION)
- TABLE 104 EUROPE: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DATA SETS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 105 EUROPE: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 106 EUROPE: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 107 EUROPE: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 108 EUROPE: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2023-2030 (USD MILLION)
- TABLE 109 EUROPE: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2023-2030 (USD MILLION)
- TABLE 110 EUROPE: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 111 EUROPE: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY HEALTHCARE PAYER, 2023-2030 (USD MILLION)
- TABLE 112 EUROPE: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY HEALTHCARE PROVIDER, 2023-2030 (USD MILLION)
- TABLE 113 GERMANY: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2023-2030 (USD MILLION)
- TABLE 114 GERMANY: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 115 GERMANY: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 116 GERMANY: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 117 GERMANY: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 118 GERMANY: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2023-2030 (USD MILLION)
- TABLE 119 GERMANY: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2023-2030 (USD MILLION)
- TABLE 120 GERMANY: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 121 GERMANY: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY HEALTHCARE PAYER, 2023-2030 (USD MILLION)
- TABLE 122 GERMANY: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY HEALTHCARE PROVIDER, 2023-2030 (USD MILLION)
- TABLE 123 UK: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2023-2030 (USD MILLION)
- TABLE 124 UK: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DATA SETS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 125 UK: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 126 UK: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 127 UK: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 128 UK: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2023-2030 (USD MILLION)
- TABLE 129 UK: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2023-2030 (USD MILLION)
- TABLE 130 UK: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 131 UK: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY HEALTHCARE PAYER, 2023-2030 (USD MILLION)
- TABLE 132 UK: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY HEALTHCARE PROVIDER, 2023-2030 (USD MILLION)
- TABLE 133 FRANCE: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2023-2030 (USD MILLION)
- TABLE 134 FRANCE: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DATA SETS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 135 FRANCE: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 136 FRANCE: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 137 FRANCE: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 138 FRANCE: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2023-2030 (USD MILLION)
- TABLE 139 FRANCE: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2023-2030 (USD MILLION)
- TABLE 140 FRANCE: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 141 FRANCE: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY HEALTHCARE PAYER, 2023-2030 (USD MILLION)
- TABLE 142 FRANCE: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY HEALTHCARE PROVIDER, 2023-2030 (USD MILLION)
- TABLE 143 ITALY: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2023-2030 (USD MILLION)
- TABLE 144 ITALY: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DATA SETS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 145 ITALY: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 146 ITALY: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 147 ITALY: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 148 ITALY: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2023-2030 (USD MILLION)
- TABLE 149 ITALY: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2023-2030 (USD MILLION)
- TABLE 150 ITALY: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 151 ITALY: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY HEALTHCARE PAYER, 2023-2030 (USD MILLION)
- TABLE 152 ITALY: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY HEALTHCARE PROVIDER, 2023-2030 (USD MILLION)
- TABLE 153 SPAIN: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2023-2030 (USD MILLION)
- TABLE 154 SPAIN: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DATA SETS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 155 SPAIN: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 156 SPAIN: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 157 SPAIN: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 158 SPAIN: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2023-2030 (USD MILLION)
- TABLE 159 SPAIN: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2023-2030 (USD MILLION)
- TABLE 160 SPAIN: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 161 SPAIN: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY HEALTHCARE PAYER, 2023-2030 (USD MILLION)
- TABLE 162 SPAIN: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY HEALTHCARE PROVIDER, 2023-2030 (USD MILLION)
- TABLE 163 REST OF EUROPE: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2023-2030 (USD MILLION)
- TABLE 164 REST OF EUROPE: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DATA SETS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 165 REST OF EUROPE: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 166 REST OF EUROPE: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 167 REST OF EUROPE: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 168 REST OF EUROPE: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2023-2030 (USD MILLION)
- TABLE 169 REST OF EUROPE: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2023-2030 (USD MILLION)
- TABLE 170 REST OF EUROPE: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 171 REST OF EUROPE: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY HEALTHCARE PAYER, 2023-2030 (USD MILLION)
- TABLE 172 REST OF EUROPE: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY HEALTHCARE PROVIDER, 2023-2030 (USD MILLION)
- TABLE 173 ASIA PACIFIC: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 174 ASIA PACIFIC: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2023-2030 (USD MILLION)
- TABLE 175 ASIA PACIFIC: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DATA SETS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 176 ASIA PACIFIC: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 177 ASIA PACIFIC: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 178 ASIA PACIFIC: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2023-2030 (USD MILLION)
- TABLE 179 ASIA PACIFIC: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2023-2030 (USD MILLION)
- TABLE 180 ASIA PACIFIC: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2023-2030 (USD MILLION)
- TABLE 181 ASIA PACIFIC: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 182 ASIA PACIFIC: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY HEALTHCARE PAYER, 2023-2030 (USD MILLION)
- TABLE 183 ASIA PACIFIC: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY HEALTHCARE PROVIDER, 2023-2030 (USD MILLION)
- TABLE 184 JAPAN: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2023-2030 (USD MILLION)
- TABLE 185 JAPAN: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DATA SETS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 186 JAPAN: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 187 JAPAN: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 188 JAPAN: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2023-2030 (USD MILLION)
- TABLE 189 JAPAN: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2023-2030 (USD MILLION)
- TABLE 190 JAPAN: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2023-2030 (USD MILLION)
- TABLE 191 JAPAN: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 192 JAPAN: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY HEALTHCARE PAYER, 2023-2030 (USD MILLION)
- TABLE 193 JAPAN: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY HEALTHCARE PROVIDER, 2023-2030 (USD MILLION)
- TABLE 194 CHINA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2023-2030 (USD MILLION)
- TABLE 195 CHINA: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DATA SETS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 196 CHINA: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 197 CHINA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 198 CHINA: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2023-2030 (USD MILLION)
- TABLE 199 CHINA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2023-2030 (USD MILLION)
- TABLE 200 CHINA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2023-2030 (USD MILLION)
- TABLE 201 CHINA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 202 CHINA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY HEALTHCARE PAYER, 2023-2030 (USD MILLION)
- TABLE 203 CHINA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY HEALTHCARE PROVIDER, 2023-2030 (USD MILLION)
- TABLE 204 INDIA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2023-2030 (USD MILLION)
- TABLE 205 INDIA: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DATA SETS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 206 INDIA: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 207 INDIA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 208 INDIA: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2023-2030 (USD MILLION)
- TABLE 209 INDIA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2023-2030 (USD MILLION)
- TABLE 210 INDIA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2023-2030 (USD MILLION)
- TABLE 211 INDIA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 212 INDIA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY HEALTHCARE PAYER, 2023-2030 (USD MILLION)
- TABLE 213 INDIA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY HEALTHCARE PROVIDER, 2023-2030 (USD MILLION)
- TABLE 214 AUSTRALIA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2023-2030 (USD MILLION)
- TABLE 215 AUSTRALIA: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DATA SETS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 216 AUSTRALIA: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 217 AUSTRALIA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 218 AUSTRALIA: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2023-2030 (USD MILLION)
- TABLE 219 AUSTRALIA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2023-2030 (USD MILLION)
- TABLE 220 AUSTRALIA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2023-2030 (USD MILLION)
- TABLE 221 AUSTRALIA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 222 AUSTRALIA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY HEALTHCARE PAYER, 2023-2030 (USD MILLION)
- TABLE 223 AUSTRALIA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY HEALTHCARE PROVIDER, 2023-2030 (USD MILLION)
- TABLE 224 SOUTH KOREA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2023-2030 (USD MILLION)
- TABLE 225 SOUTH KOREA: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DATA SETS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 226 SOUTH KOREA: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 227 SOUTH KOREA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 228 SOUTH KOREA: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2023-2030 (USD MILLION)
- TABLE 229 SOUTH KOREA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2023-2030 (USD MILLION)
- TABLE 230 SOUTH KOREA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2023-2030 (USD MILLION)
- TABLE 231 SOUTH KOREA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 232 SOUTH KOREA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY HEALTHCARE PAYER, 2023-2030 (USD MILLION)
- TABLE 233 SOUTH KOREA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY HEALTHCARE PROVIDER, 2023-2030 (USD MILLION)
- TABLE 234 REST OF ASIA PACIFIC: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2023-2030 (USD MILLION)
- TABLE 235 REST OF ASIA PACIFIC: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DATA SETS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 236 REST OF ASIA PACIFIC: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 237 REST OF ASIA PACIFIC: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 238 REST OF ASIA PACIFIC: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2023-2030 (USD MILLION)
- TABLE 239 REST OF ASIA PACIFIC: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2023-2030 (USD MILLION)
- TABLE 240 REST OF ASIA PACIFIC: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2023-2030 (USD MILLION)
- TABLE 241 REST OF ASIA PACIFIC: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 242 REST OF ASIA PACIFIC: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY HEALTHCARE PAYER, 2023-2030 (USD MILLION)
- TABLE 243 REST OF ASIA PACIFIC: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY HEALTHCARE PROVIDER, 2023-2030 (USD MILLION)
- TABLE 244 LATIN AMERICA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 245 LATIN AMERICA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2023-2030 (USD MILLION)
- TABLE 246 LATIN AMERICA: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DATA SETS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 247 LATIN AMERICA: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 248 LATIN AMERICA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 249 LATIN AMERICA: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2023-2030 (USD MILLION)
- TABLE 250 LATIN AMERICA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2023-2030 (USD MILLION)
- TABLE 251 LATIN AMERICA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2023-2030 (USD MILLION)
- TABLE 252 LATIN AMERICA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 253 LATIN AMERICA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY HEALTHCARE PAYER, 2023-2030 (USD MILLION)
- TABLE 254 LATIN AMERICA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY HEALTHCARE PROVIDER, 2023-2030 (USD MILLION)
- TABLE 255 BRAZIL: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2023-2030 (USD MILLION)
- TABLE 256 BRAZIL: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DATA SETS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 257 BRAZIL: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 258 BRAZIL: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 259 BRAZIL: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2023-2030 (USD MILLION)
- TABLE 260 BRAZIL: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2023-2030 (USD MILLION)
- TABLE 261 BRAZIL: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2023-2030 (USD MILLION)
- TABLE 262 BRAZIL: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 263 BRAZIL: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY HEALTHCARE PAYER, 2023-2030 (USD MILLION)
- TABLE 264 BRAZIL: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY HEALTHCARE PROVIDER, 2023-2030 (USD MILLION)
- TABLE 265 MEXICO: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2023-2030 (USD MILLION)
- TABLE 266 MEXICO: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DATA SETS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 267 MEXICO: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 268 MEXICO: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 269 MEXICO: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2023-2030 (USD MILLION)
- TABLE 270 MEXICO: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2023-2030 (USD MILLION)
- TABLE 271 MEXICO: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2023-2030 (USD MILLION)
- TABLE 272 MEXICO: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 273 MEXICO: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY HEALTHCARE PAYER, 2023-2030 (USD MILLION)
- TABLE 274 MEXICO: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY HEALTHCARE PROVIDER, 2023-2030 (USD MILLION)
- TABLE 275 REST OF LATIN AMERICA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2023-2030 (USD MILLION)
- TABLE 276 REST OF LATIN AMERICA: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DATA SETS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 277 REST OF LATIN AMERICA: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 278 REST OF LATIN AMERICA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 279 REST OF LATIN AMERICA: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2023-2030 (USD MILLION)
- TABLE 280 REST OF LATIN AMERICA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2023-2030 (USD MILLION)
- TABLE 281 REST OF LATIN AMERICA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2023-2030 (USD MILLION)
- TABLE 282 REST OF LATIN AMERICA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 283 REST OF LATIN AMERICA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY HEALTHCARE PAYER, 2023-2030 (USD MILLION)
- TABLE 284 REST OF LATIN AMERICA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY HEALTHCARE PROVIDER, 2023-2030 (USD MILLION)
- TABLE 285 MIDDLE EAST & AFRICA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 286 MIDDLE EAST & AFRICA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2023-2030 (USD MILLION)
- TABLE 287 MIDDLE EAST & AFRICA: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DATA SETS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 288 MIDDLE EAST & AFRICA: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 289 MIDDLE EAST & AFRICA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 290 MIDDLE EAST & AFRICA: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2023-2030 (USD MILLION)
- TABLE 291 MIDDLE EAST & AFRICA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2023-2030 (USD MILLION)
- TABLE 292 MIDDLE EAST & AFRICA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2023-2030 (USD MILLION)
- TABLE 293 MIDDLE EAST & AFRICA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 294 MIDDLE EAST & AFRICA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY HEALTHCARE PAYER, 2023-2030 (USD MILLION)
- TABLE 295 MIDDLE EAST & AFRICA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY HEALTHCARE PROVIDER, 2023-2030 (USD MILLION)
- TABLE 296 GCC COUNTRIES: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 297 GCC COUNTRIES: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2023-2030 (USD MILLION)
- TABLE 298 GCC COUNTRIES: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DATA SETS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 299 GCC COUNTRIES: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 300 GCC COUNTRIES: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 301 GCC COUNTRIES: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2023-2030 (USD MILLION)
- TABLE 302 GCC COUNTRIES: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2023-2030 (USD MILLION)
- TABLE 303 GCC COUNTRIES: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2023-2030 (USD MILLION)
- TABLE 304 GCC COUNTRIES: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 305 GCC COUNTRIES: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY HEALTHCARE PAYER, 2023-2030 (USD MILLION)
- TABLE 306 GCC COUNTRIES: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY HEALTHCARE PROVIDER, 2023-2030 (USD MILLION)
- TABLE 307 SAUDI ARABIA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2023-2030 (USD MILLION)
- TABLE 308 SAUDI ARABIA: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DATA SETS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 309 SAUDI ARABIA: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 310 SAUDI ARABIA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 311 SAUDI ARABIA: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2023-2030 (USD MILLION)
- TABLE 312 SAUDI ARABIA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2023-2030 (USD MILLION)
- TABLE 313 SAUDI ARABIA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2023-2030 (USD MILLION)
- TABLE 314 SAUDI ARABIA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 315 SAUDI ARABIA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY HEALTHCARE PAYER, 2023-2030 (USD MILLION)
- TABLE 316 SAUDI ARABIA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY HEALTHCARE PROVIDER, 2023-2030 (USD MILLION)
- TABLE 317 UAE: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2023-2030 (USD MILLION)
- TABLE 318 UAE: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DATA SETS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 319 UAE: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 320 UAE: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 321 UAE: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2023-2030 (USD MILLION)
- TABLE 322 UAE: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2023-2030 (USD MILLION)
- TABLE 323 UAE: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2023-2030 (USD MILLION)
- TABLE 324 UAE: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 325 UAE: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY HEALTHCARE PAYER, 2023-2030 (USD MILLION)
- TABLE 326 UAE: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY HEALTHCARE PROVIDER, 2023-2030 (USD MILLION)
- TABLE 327 REST OF GCC: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2023-2030 (USD MILLION)
- TABLE 328 REST OF GCC: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DATA SETS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 329 REST OF GCC: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 330 REST OF GCC: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 331 REST OF GCC: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2023-2030 (USD MILLION)
- TABLE 332 REST OF GCC: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2023-2030 (USD MILLION)
- TABLE 333 REST OF GCC: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2023-2030 (USD MILLION)
- TABLE 334 REST OF GCC: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 335 REST OF GCC: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY HEALTHCARE PAYER, 2023-2030 (USD MILLION)
- TABLE 336 REST OF GCC: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY HEALTHCARE PROVIDER, 2023-2030 (USD MILLION)
- TABLE 337 SOUTH AFRICA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2023-2030 (USD MILLION)
- TABLE 338 SOUTH AFRICA: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DATA SETS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 339 SOUTH AFRICA: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 340 SOUTH AFRICA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 341 SOUTH AFRICA: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2023-2030 (USD MILLION)
- TABLE 342 SOUTH AFRICA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2023-2030 (USD MILLION)
- TABLE 343 SOUTH AFRICA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2023-2030 (USD MILLION)
- TABLE 344 SOUTH AFRICA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 345 SOUTH AFRICA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY HEALTHCARE PAYER, 2023-2030 (USD MILLION)
- TABLE 346 SOUTH AFRICA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY HEALTHCARE PROVIDER, 2023-2030 (USD MILLION)
- TABLE 347 REST OF MIDDLE EAST & AFRICA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2023-2030 (USD MILLION)
- TABLE 348 REST OF MIDDLE EAST & AFRICA: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DATA SETS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 349 REST OF MIDDLE EAST & AFRICA: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 350 REST OF MIDDLE EAST & AFRICA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 351 REST OF MIDDLE EAST & AFRICA: REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2023-2030 (USD MILLION)
- TABLE 352 REST OF MIDDLE EAST & AFRICA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2023-2030 (USD MILLION)
- TABLE 353 REST OF MIDDLE EAST & AFRICA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2023-2030 (USD MILLION)
- TABLE 354 REST OF MIDDLE EAST & AFRICA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 355 REST OF MIDDLE EAST & AFRICA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY HEALTHCARE PAYER, 2023-2030 (USD MILLION)
- TABLE 356 REST OF MIDDLE EAST & AFRICA: REAL WORLD EVIDENCE SOLUTIONS MARKET, BY HEALTHCARE PROVIDER, 2023-2030 (USD MILLION)
- TABLE 357 OVERVIEW OF STRATEGIES ADOPTED BY KEY PLAYERS, JANUARY 2022-JULY 2025
- TABLE 358 REAL WORLD EVIDENCE SOLUTIONS MARKET: DEGREE OF COMPETITION
- TABLE 359 REGIONAL FOOTPRINT
- TABLE 360 COMPONENT FOOTPRINT
- TABLE 361 END USER FOOTPRINT
- TABLE 362 APPLICATION FOOTPRINT
- TABLE 363 REAL WORLD EVIDENCE SOLUTIONS MARKET: DETAILED LIST OF KEY STARTUPS/SMES
- TABLE 364 REAL WORLD EVIDENCE SOLUTIONS MARKET: COMPETITIVE BENCHMARKING OF KEY STARTUPS/SMES
- TABLE 365 REAL WORLD EVIDENCE SOLUTIONS MARKET: PRODUCT LAUNCHES/ENHANCEMENTS, JANUARY 2022-JULY 2025
- TABLE 366 REAL WORLD EVIDENCE SOLUTIONS MARKET: DEALS, JANUARY 2022-JULY 2025
- TABLE 367 REAL WORLD EVIDENCE SOLUTIONS MARKET: OTHER DEVELOPMENTS, JANUARY 2022-JULY 2025
- TABLE 368 IQVIA: COMPANY OVERVIEW
- TABLE 369 IQVIA: PRODUCTS OFFERED
- TABLE 370 IQVIA: DEALS, JANUARY 2022-JULY 2025
- TABLE 371 MERATIVE: COMPANY OVERVIEW
- TABLE 372 MERATIVE: PRODUCTS OFFERED
- TABLE 373 MERATIVE: DEALS, JANUARY 2022-JULY 2025
- TABLE 374 MERATIVE: OTHER DEVELOPMENTS, JANUARY 2022-JULY 2025
- TABLE 375 OPTUM, INC.: COMPANY OVERVIEW
- TABLE 376 OPTUM, INC.: PRODUCTS OFFERED
- TABLE 377 OPTUM, INC.: PRODUCT LAUNCHES/DEVELOPMENTS, JANUARY 2022-JULY 2025
- TABLE 378 OPTUM, INC. DEALS, JANUARY 2022-JULY 2025
- TABLE 379 ICON PLC: COMPANY OVERVIEW
- TABLE 380 ICON PLC: PRODUCTS OFFERED
- TABLE 381 ICON PLC: PRODUCT LAUNCHES/DEVELOPMENTS, JANUARY 2022-JULY 2025
- TABLE 382 ICON PLC: DEALS, JANUARY 2022-JULY 2025
- TABLE 383 SYNEOS HEALTH: COMPANY OVERVIEW
- TABLE 384 SYNEOS HEALTH: PRODUCTS OFFERED
- TABLE 385 SYNEOS HEALTH: DEALS, JANUARY 2022-JULY 2025
- TABLE 386 PAREXEL INTERNATIONAL (MA) CORPORATION: COMPANY OVERVIEW
- TABLE 387 PAREXEL INTERNATIONAL (MA) CORPORATION: PRODUCTS OFFERED
- TABLE 388 PAREXEL INTERNATIONAL (MA) CORPORATION: PRODUCT LAUNCHES/DEVELOPMENTS, JANUARY 2022-JULY 2025
- TABLE 389 PAREXEL INTERNATIONAL (MA) CORPORATION: DEALS, JANUARY 2022-JULY 2025
- TABLE 390 FLATIRON HEALTH: COMPANY OVERVIEW
- TABLE 391 FLATIRON HEALTH: PRODUCTS OFFERED
- TABLE 392 FLATIRON HEALTH: PRODUCT LAUNCHES/DEVELOPMENTS, JANUARY 2022-JULY 2025
- TABLE 393 FLATIRON HEALTH: DEALS, JANUARY 2022-JULY 2025
- TABLE 394 FORTREA: COMPANY OVERVIEW
- TABLE 395 FORTREA: PRODUCTS OFFERED
- TABLE 396 FORTREA: PRODUCT LAUNCHES/DEVELOPMENTS, JANUARY 2022-JULY 2025
- TABLE 397 FORTREA: DEALS, JANUARY 2022-JULY 2025
- TABLE 398 ORACLE: COMPANY OVERVIEW
- TABLE 399 ORACLE: PRODUCTS OFFERED
- TABLE 400 ORACLE: PRODUCT LAUNCHES/DEVELOPMENTS, JANUARY 2022-JULY 2025
- TABLE 401 ORACLE: DEALS, JANUARY 2022-JULY 2025
- TABLE 402 ELEVANCE HEALTH: COMPANY OVERVIEW
- TABLE 403 ELEVANCE HEALTH: PRODUCTS OFFERED
- TABLE 404 SAS INSTITUTE INC.: COMPANY OVERVIEW
- TABLE 405 SAS INSTITUTE INC.: PRODUCTS OFFERED
- TABLE 406 SAS INSTITUTE INC.: PRODUCT LAUNCHES/DEVELOPMENTS, JANUARY 2022-JULY 2025
- TABLE 407 AETION, INC.: COMPANY OVERVIEW
- TABLE 408 AETION, INC.: PRODUCTS OFFERED
- TABLE 409 AETION, INC.: PRODUCT LAUNCHES/DEVELOPMENTS, JANUARY 2022-JULY 2025
- TABLE 410 AETION, INC.: DEALS, JANUARY 2022-JULY 2025
- TABLE 411 TRINETX, LLC: COMPANY OVERVIEW
- TABLE 412 TRINETX, LLC: PRODUCTS OFFERED
- TABLE 413 TRINETX, LLC: PRODUCT LAUNCHES/DEVELOPMENTS, JANUARY 2022-JULY 2025
- TABLE 414 TRINETX, LLC: DEALS, JANUARY 2022-JULY 2025
- TABLE 415 TRINITY: COMPANY OVERVIEW
- TABLE 416 TRINITY: PRODUCTS OFFERED
- TABLE 417 TRINITY: DEALS, JANUARY 2022-JULY 2025
- TABLE 418 MEDIDATA: COMPANY OVERVIEW
- TABLE 419 MEDIDATA: PRODUCTS OFFERED
- TABLE 420 MEDIDATA: PRODUCT LAUNCHES/DEVELOPMENTS, JANUARY 2022-JULY 2025
- TABLE 421 MEDIDATA: DEALS, JANUARY 2022-JULY 2025
- TABLE 422 COGNIZANT TECHNOLOGY SOLUTIONS CORPORATION: COMPANY OVERVIEW
- TABLE 423 COGNIZANT TECHNOLOGY SOLUTIONS CORPORATION: PRODUCTS OFFERED
- TABLE 424 CEGEDIM HEALTH DATA: COMPANY OVERVIEW
- TABLE 425 CEGEDIM HEALTH DATA: PRODUCTS OFFERED
- TABLE 426 CEGEDIM HEALTH DATA: PRODUCT LAUNCHES/DEVELOPMENTS, JANUARY 2022-JULY 2025
- TABLE 427 CEGEDIM HEALTH DATA: DEALS, JANUARY 2022-JULY 2025
- TABLE 428 VERANTOS: COMPANY OVERVIEW
- TABLE 429 VERANTOS: PRODUCTS OFFERED
- TABLE 430 VERANTOS: PRODUCT LAUNCHES/DEVELOPMENTS, JANUARY 2022-JULY 2025
- TABLE 431 VERANTOS: DEALS, JANUARY 2022-JULY 2025
- TABLE 432 MEDPACE: COMPANY OVERVIEW
- TABLE 433 MEDPACE: PRODUCTS OFFERED
- TABLE 434 TATA CONSULTANCY SERVICES LIMITED: COMPANY OVERVIEW
- TABLE 435 TATA CONSULTANCY SERVICES LIMITED: PRODUCTS OFFERED
- TABLE 436 TATA CONSULTANCY SERVICES LIMITED: DEALS, JANUARY 2022-JULY 2025
- TABLE 437 HEALTHVERITY, INC.: COMPANY OVERVIEW
- TABLE 438 OM1: COMPANY OVERVIEW
- TABLE 439 OPEN HEALTH: COMPANY OVERVIEW
- TABLE 440 TEMPUS: COMPANY OVERVIEW
- TABLE 441 QUANTZIG: COMPANY OVERVIEW
List of Figures
- FIGURE 1 RESEARCH DESIGN
- FIGURE 2 PRIMARY SOURCES
- FIGURE 3 BREAKDOWN OF PRIMARY INTERVIEWS: BY COMPANY TYPE, DESIGNATION, AND REGION
- FIGURE 4 RESEARCH METHODOLOGY: HYPOTHESIS BUILDING
- FIGURE 5 SUPPLY-SIDE MARKET SIZE ESTIMATION: REVENUE SHARE ANALYSIS
- FIGURE 6 BOTTOM-UP APPROACH
- FIGURE 7 TOP-DOWN APPROACH
- FIGURE 8 CAGR PROJECTIONS FROM ANALYSIS OF MARKET DYNAMICS, 2024-2030
- FIGURE 9 CAGR PROJECTIONS: SUPPLY-SIDE ANALYSIS
- FIGURE 10 DATA TRIANGULATION METHODOLOGY
- FIGURE 11 REAL WORLD EVIDENCE SOLUTIONS MARKET, BY COMPONENT, 2025 VS. 2030 (USD MILLION)
- FIGURE 12 REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DATA SETS, BY TYPE, 2025 VS. 2030 (USD MILLION)
- FIGURE 13 REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DISPARATE DATA SETS, BY TYPE, 2025 VS. 2030 (USD MILLION)
- FIGURE 14 REAL WORLD EVIDENCE SOLUTIONS MARKET, BY APPLICATION, 2025 VS. 2030 (USD MILLION)
- FIGURE 15 REAL WORLD EVIDENCE SOLUTIONS MARKET FOR DRUG DEVELOPMENT & APPROVALS, BY THERAPEUTIC AREA, 2025 VS. 2030 (USD MILLION)
- FIGURE 16 REAL WORLD EVIDENCE SOLUTIONS MARKET, BY REVENUE MODEL, 2025 VS. 2030 (USD MILLION)
- FIGURE 17 REAL WORLD EVIDENCE SOLUTIONS MARKET, BY DEPLOYMENT MODE, 2025 VS. 2030 (USD MILLION)
- FIGURE 18 REAL WORLD EVIDENCE SOLUTIONS MARKET, BY END USER, 2025 VS. 2030 (USD MILLION)
- FIGURE 19 REAL WORLD EVIDENCE SOLUTIONS MARKET: GEOGRAPHIC SNAPSHOT
- FIGURE 20 RISING GERIATRIC POPULATION AND SUBSEQUENT INCREASE IN PREVALENCE OF CHRONIC DISEASES TO DRIVE MARKET
- FIGURE 21 PHARMACEUTICAL & MEDICAL DEVICE COMPANIES SEGMENT AND CHINA ACCOUNTED FOR SIGNIFICANT SHARE IN 2024
- FIGURE 22 INDIA TO REGISTER HIGHEST GROWTH DURING FORECAST PERIOD
- FIGURE 23 NORTH AMERICA TO DOMINATE MARKET DURING FORECAST PERIOD
- FIGURE 24 EMERGING ECONOMIES TO REGISTER SIGNIFICANT GROWTH DURING FORECAST PERIOD
- FIGURE 25 REAL WORLD EVIDENCE SOLUTIONS MARKET: DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES
- FIGURE 26 NUMBER OF CLINICAL TRIALS ACROSS REGIONS, 2022-2024
- FIGURE 27 RECOMMENDED INVESTMENT MODEL FOR RWD AND RWE
- FIGURE 28 RWE ANALYTICS APPROACH: INTERNAL VS. OUTSOURCED
- FIGURE 29 ECOSYSTEM ANALYSIS
- FIGURE 30 REAL WORLD EVIDENCE SOLUTIONS MARKET: VALUE CHAIN ANALYSIS
- FIGURE 31 REVENUE SHIFT IN REAL WORLD EVIDENCE SOLUTIONS MARKET
- FIGURE 32 PORTER'S FIVE FORCES ANALYSIS
- FIGURE 33 JURISDICTION AND TOP APPLICANT ANALYSIS
- FIGURE 34 TOP APPLICANTS AND OWNERS (COMPANIES/INSTITUTIONS), JANUARY 2015 TO JULY 2025
- FIGURE 35 INFLUENCE OF STAKEHOLDERS ON BUYING PROCESS FOR KEY APPLICATIONS
- FIGURE 36 KEY BUYING CRITERIA FOR KEY APPLICATIONS
- FIGURE 37 INVESTMENT & FUNDING SCENARIO
- FIGURE 38 MARKET POTENTIAL OF AI/GENERATIVE AI IN ENHANCING REAL WORLD EVIDENCE SOLUTIONS ACROSS INDUSTRIES
- FIGURE 39 IMPACT OF GENERATIVE AI ON INTERCONNECTED AND ADJACENT MARKET ECOSYSTEM
- FIGURE 40 NORTH AMERICA: REAL WORLD EVIDENCE SOLUTIONS MARKET SNAPSHOT
- FIGURE 41 ASIA PACIFIC: REAL WORLD EVIDENCE SOLUTIONS MARKET SNAPSHOT
- FIGURE 42 REVENUE ANALYSIS OF KEY PLAYERS, 2020-2024 (USD MILLION)
- FIGURE 43 MARKET SHARE ANALYSIS OF KEY PLAYERS, 2024
- FIGURE 44 REAL WORLD EVIDENCE SOLUTIONS MARKET: COMPANY EVALUATION MATRIX (KEY PLAYERS), 2024
- FIGURE 45 REAL WORLD EVIDENCE SOLUTIONS MARKET: COMPANY FOOTPRINT
- FIGURE 46 REAL WORLD EVIDENCE SOLUTIONS MARKET: COMPANY EVALUATION MATRIX (STARTUPS/SMES), 2024
- FIGURE 47 BRAND/PRODUCT COMPARISON
- FIGURE 48 EV/EBITDA OF KEY VENDORS
- FIGURE 49 YEAR-TO-DATE (YTD) PRICE TOTAL RETURN AND 5-YEAR STOCK BETA OF REAL WORLD EVIDENCE SOLUTIONS, 2024
- FIGURE 50 IQVIA: COMPANY SNAPSHOT (2024)
- FIGURE 51 OPTUM, INC.: COMPANY SNAPSHOT (2024)
- FIGURE 52 ICON PLC: COMPANY SNAPSHOT (2024)
- FIGURE 53 FORTREA: COMPANY SNAPSHOT (2024)
- FIGURE 54 ORACLE: COMPANY SNAPSHOT (2024)
- FIGURE 55 ELEVANCE HEALTH: COMPANY SNAPSHOT (2024)
- FIGURE 56 COGNIZANT TECHNOLOGY SOLUTIONS CORPORATION: COMPANY SNAPSHOT (2024)
- FIGURE 57 CEGEDIM HEALTH DATA: COMPANY SNAPSHOT (2024)
- FIGURE 58 MEDPACE: COMPANY SNAPSHOT (2024)
- FIGURE 59 TATA CONSULTANCY SERVICES LIMITED.: COMPANY SNAPSHOT (2024)












