|
市场调查报告书
商品编码
1349826
HER2-low 转移性乳癌 (mBC) 市场:初步研究 (KOL 见解) - 市场情报 - 流行病学和 2034 年市场预测HER2-Low Metastatic Breast Cancer (mBC) | Primary Research (KOL's Insight) | Market Intelligence | Epidemiology & Market Forecast-2034 |
|||||||
HER2低转移性乳癌(mBC)市场以化疗、内分泌治疗和曲妥珠单抗deruxtecan(Enhertu)等标靶治疗为主。到 2034 年,新兴疗法的采用将成为一个重大转折点,将为 HER2 低转移性乳癌药物市场带来巨大变化。在调查国家(美国、法国、德国、义大利、西班牙、英国和日本),用于治疗 HER2 低转移性乳癌的新兴疗法的销售额预计在 2020 年至 2034 年的研究期间将出现强劲增长。预料到的。
HER2 低表达转移性乳癌的治疗似乎正在迅速发展。最近的临床试验表明,CDK4/6抑制剂与内分泌治疗的组合作为标准一线治疗是有效的。此外,PI3K抑制剂和AKT抑制剂的使用也在临床试验中进行研究,可能在不久的将来提供进一步的治疗选择。"

HER2 低表达的转移性乳癌代表了乳癌认知和治疗的重大转变。历史上,乳癌依 HER2 表现量分为 HER2 阳性或 HER2 阴性。然而,目前超过50%的乳癌被定义为“人类表皮生长因子受体2(HER2)低表达乳癌(BC)”,HER2免疫组织化学(IHC)评分为+1或+2,且萤光原位杂交(FISH)测试呈阴性。IHC/ISH 是目前用于定义 HER2 表达的唯一标准技术。最近的证据显示 HER2 低表达乳癌可能是具有治疗意义的目标亚群。
预计到 2034 年,G7 国家 HER2 低转移性乳癌病例总数将显着增加。据估计,到 2034 年,美国将成为 HER2 低转移性乳癌发生率最高的国家。在欧盟五国中,德国的 HER2 低转移性乳癌病例数最多,其次是英国、法国、义大利和西班牙。据报道,日本的治疗病例数仅次于美国、德国和英国。
根据SEER资料库,预计2022年美国将新诊断出287,850例女性乳癌,其中约50-60%的新诊断乳癌患者患有HER2低转移性乳癌。确诊。
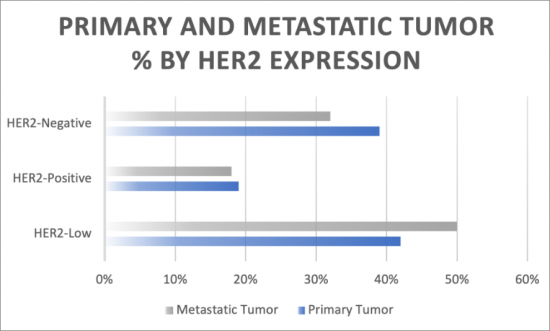
HER2低表达转移性乳癌治疗市场预计将在研究期间(即2020年至2034年)经历高速成长。与欧洲五国和日本相比,美国的市占率最高。
本报告检视了全球 HER2 低转移性乳癌 (mBC) 市场,并提供了市场现状以及病例量趋势、患者趋势、竞争产品市场定位和市场机会。
目录
执行摘要
HER2低转移性乳癌疾病背景
- HER2低转移性乳癌的定义
- 原因和症状
- 病理生理学
- 导致乳癌 HER2 低表现的因素
HER2低转移性乳癌的诊断
流行病学和患者群体
- 主要发现
- 方法和资料来源
- HER2 低转移性乳癌流行病学和模型参数的主要来源
- 美国
- 德国
- 法国
- 意大利
- 西班牙
- 英国
- 日本
- 目前的治疗方法和医疗实践
- 非处方疗法
- 未满足的需求
- 新的治疗方法
- 产品分析
- 发布时间表和主要市场事件
- 价格和赎回
- KOL 洞察(美国、欧洲、日本)
- 未来的治疗范式
- 目前和未来治疗的年度费用
- HER2低转移性乳癌晚期治疗的策略考虑
- 市场展望
- 主要药品市场HER2低转移性乳癌药物销售(2020-2034年)
- 按治疗方法划分的 HER2 低转移性乳癌患者百分比
- 国家市场预测
- 美国
- 德国
- 法国
- 西班牙
- 英国
- 日本
- 市场驱动因素与限制因素
- 附录
The HER2-Low Metastatic Breast Cancer (mBC) market is hugely contributed by chemotherapy, endocrine therapy and targeted therapy such as Trastuzumab deruxtecan (Enhertu). By 2034, the uptake of novel emerging therapies will serve as a major breakpoint to get a drastic change in the HER2-Low Metastatic Breast Cancer therapeutics market. The sales of the emerging therapies for the treatment of HER2-Low Metastatic Breast Cancer in the study countries (United States, France, Germany, Italy, Spain, United Kingdom and Japan) will experience high growth over the 2020-2034 study period.
"Experts believe that the treatment armamentarium for HER2-low metastatic breast cancer is rapidly evolving. Recent clinical trials have demonstrated the efficacy of CDK4/6 inhibitors in combination with endocrine therapy as a standard first-line treatment option. Additionally, the use of PI3K inhibitors and AKT inhibitors is being explored in clinical trials and may provide further treatment options in the near future."
The introduction of HER2-low metastatic breast cancer represents a significant shift in the understanding and treatment of breast cancer. Historically, breast cancers were categorized as either HER2-positive or HER2-negative based on the level of HER2 expression. However, more than 50% of breast cancers are currently defined as "Human epidermal growth factor receptor 2 (HER2) low breast cancer (BC)", with HER2 immunohistochemistry (IHC) scores of +1 or +2 with a negative fluorescence in situ hybridization (FISH) test. IHC/ISH is the only standard technique currently applied to define HER2 expression. Recent evidence suggests that HER2-low breast cancer can be a targetable subgroup with potential therapeutic implications.

Mellalta's HER2-Low Metastatic Breast Cancer Report– Market Summary
| Report Attributes | Details |
| Key Market Players: | Jiangsu HengRui Medicine; Duality Biologics; Yantai Rongchang Pharmaceutical; Gilead Sciences; AstraZeneca/Daiichi Sankyo. |
| Forecast Period: | 2020-2034. |
| Countries Covered: | US, France, Germany, Italy, Spain, UK, China and Japan. |
| Current SOC: | Chemotherapy; Endocrine Therapies; Targeted Therapies. |
| Future SOC: | Targeted Therapies; Combination Approach. |
| Key Unmet Need: | Improved HER2 Assessment; Limited Treatment Option. |
| Key Clinical Insights: | Emergence of HER2-low breast cancer has led to a significant change in treatment algorithms for patients with breast cancer. The introduction of targeted therapies like T-DXd offers new treatment options for patients who were previously considered to have limited options. |
| Provider-Patient (PPP) Perspective: | Cost-effectiveness of treatment; Better Diagnostic Identification; Access to appropriate treatments and improved options. |
Mellalta's HER2-Low Metastatic Breast Cancer Report – Epidemiology
The total incident cases of HER2-Low Metastatic Breast Cancer in the G7 countries are anticipated to increase by a significant number of cases by 2034 for the study period (2020-2034). As per estimates, the United States will present with the highest incidence of HER2-Low Metastatic Breast Cancer cases in 2034. Among the EU5, Germany had the highest HER2-Low Metastatic Breast Cancer cases, followed by the UK, France, Italy, and Spain. Japan is reported to have the highest number of treated cases after the United States, Germany, and the UK.
According to SEER database estimates 287,850 new cases of female breast cancer will be diagnosed in 2022 in the U.S. Out of the newly diagnosed breast cancer patient, roughly 50-60% of patients is now diagnosed with HER2 Low metastatic Breast Cancer.

Mellalta's HER2-Low Metastatic Breast Cancer Report – Current Market Size & Forecast Trends
The HER2-Low Metastatic Breast Cancer therapeutics market is expected to experience high growth throughout our study period (i.e., 2020 to 2034). The United States captured the highest market share as compared to the European 5 countries and Japan.
The current standard of care is limited to chemotherapy, endocrine and targeted therapy. In 2022, Enhertu (fam-trastuzumab-deruxtecan-nxki), an IV infusion is approved as the first targeted therapy for the treatment of patients with unresectable or metastatic HER2-low breast cancer in the United States and in 2023 in the European Union. This shift in treatment options has significant implications for the market. The changing treatment landscape has created new opportunities in the market for more companies to develop and commercialize the use of ADCs in HER2-low tumours. Additionally, the entrance of ADCs like Enhertu in the treatment paradigm of HER2-low breast cancer will also pave the way for diagnostic companies to develop the specific test for the identification of HER2-low breast cancer patient pool, which will be in turn increase the treated patient pool and will drive the market of HER2 low breast cancer significantly.
While the results of DESTINY-Breast04 study led to the breakthrough approval of Enhertu in the HER2 low breast cancer treatment space, it also changed the prescribing pattern among clinician's posing significant obstacles. These challenges include raising clinician awareness through education, updating practice guidelines, managing increasing treatment complexity, and addressing a lack of data surrounding the complex characteristics and temporal heterogeneity of HER2-low tumors.
In the 2024-2034 forecast period, there will be tremendous growth and shift in the therapeutic market with the launch of noble emerging therapies like Trastuzumab rezetecan (Jiangsu HengRui Medicine), DB-1303 (Duality Biologics), Disitamab vedotin (Yantai Rongchang Pharmaceutical), Sacituzumab Govitecan (Gilead Sciences), Datopotamab deruxtecan (AstraZeneca/Daiichi Sankyo), MRG002 (Miracogen), and more. We expect a greater uptake of the new therapies which will result in better treatment outcomes for HER2-Low Metastatic Breast Cancer market space. The launch of these upcoming therapies will drive the highly competitive therapeutic market in the coming time.
Questions Answered:
- What is the size of clinically and commercially relevant drug-treatable HER2 low BC populations, and how will drug-treatment rates of HER2 change over time?
- What is the expected market impact of recent drug approval such as Enhertu in the treatment landscape of HER2-low metastatic BC?
- Potential challenges and opportunities in implementing targeted therapies for HER2-low breast cancer.
- What are the most promising agents in the pipeline, and how will they shape the future of this therapy market?
- What key drivers and constraints will affect the HER2-low metastatic breast cancer therapy market over the forecast period?
Report Highlights:
- HER2-Low Metastatic Breast Cancer (mBC) – Current Market Trends
- HER2-Low Metastatic Breast Cancer (mBC) – Current & Forecasted Cases across the G7 Countries
- HER2-Low Metastatic Breast Cancer (mBC) – Market Opportunities and Sales Potential for Agents
- HER2-Low Metastatic Breast Cancer (mBC) – Patient-based Market Forecast to 2034
- HER2-Low Metastatic Breast Cancer (mBC) – Untapped Business Opportunities
- HER2-Low Metastatic Breast Cancer (mBC) – Product Positioning Vis-a-vis Competitors' Products
- HER2-Low Metastatic Breast Cancer (mBC) – KOLs Insight
Table of Content
Executive Summary
- Key Findings
- Key Market Challenges and Opportunities
- What Do the Experts Say?
HER2-Low Metastatic Breast Cancer Disease Background
- HER2-Low Metastatic Breast Cancer Definition
- Cause & Symptoms
- Pathophysiology
- Factors contributing to the HER2-Low Expression in Breast Cancer
HER2-Low Metastatic Breast Cancer–Diagnosis
- HER2 Assessment with Immunohistochemistry (IHC) and In Situ Hybridization (ISH) (ASCO/CAP Guidelines)
Epidemiology and Patient Populations
- Key Findings
- Methods and data Sources
- Country Specific Incident cases of Metastatic Breast Cancer (US, Germany, France, Italy, Spain, UK, and Japan)
- Country Specific Incident cases of HER2-Low Metastatic Breast Cancer
- Country Specific Treated cases of HER2-Low Metastatic Breast Cancer
- Key Sources for HER2-Low Metastatic Breast Cancer Epidemiology and Model Parameters
- United States
- United States Incident cases of Metastatic Breast Cancer (US, Germany, France, Italy, Spain, UK, and Japan)
- United States Incident cases of HER2-Low Metastatic Breast Cancer
- United States Treated cases of HER2-Low Metastatic Breast Cancer
- Germany
- Germany Incident cases of Metastatic Breast Cancer (US, Germany, France, Italy, Spain, UK, and Japan)
- Germany Incident cases of HER2-Low Metastatic Breast Cancer
- Germany Treated cases of HER2-Low Metastatic Breast Cancer
- France
- France Incident cases of Metastatic Breast Cancer (US, Germany, France, Italy, Spain, UK, and Japan)
- France Incident cases of HER2-Low Metastatic Breast Cancer
- France Treated cases of HER2-Low Metastatic Breast Cancer
- Italy
- Italy Incident cases of Metastatic Breast Cancer (US, Germany, France, Italy, Spain, UK, and Japan)
- Italy Incident cases of HER2-Low Metastatic Breast Cancer
- Italy Treated cases of HER2-Low Metastatic Breast Cancer
- Spain
- Spain Incident cases of Metastatic Breast Cancer (US, Germany, France, Italy, Spain, UK, and Japan)
- Spain Incident cases of HER2-Low Metastatic Breast Cancer
- Spain Treated cases of HER2-Low Metastatic Breast Cancer
- United Kingdom
- United Kingdom Incident cases of Metastatic Breast Cancer (US, Germany, France, Italy, Spain, UK, and Japan)
- United Kingdom Incident cases of HER2-Low Metastatic Breast Cancer
- United Kingdom Treated cases of HER2-Low Metastatic Breast Cancer
- Japan
- Japan Incident cases of Metastatic Breast Cancer (US, Germany, France, Italy, Spain, UK, and Japan)
- Japan Incident cases of HER2-Low Metastatic Breast Cancer
- Japan Treated cases of HER2-Low Metastatic Breast Cancer
- United States
- Current Therapy and Medical Practice
- Key Findings
- Treatment Algorithm
- Marketed Therapy
- ENHERTU (AstraZeneca/Daiichi Sankyo)
- Product Profile
- Clinical Development
- Market & Sales Opportunity Forecasted to 2034
- ENHERTU (AstraZeneca/Daiichi Sankyo)
- Unmet Needs
- Emerging Therapy
- Key Findings
- Pipeline Overview
- Notable Developments in the HER2-Low Metastatic Breast Cancer space
- Product Analysis
- Trastuzumab rezetecan (Jiangsu HengRui Medicine)
- Product Profile
- Clinical Development
- Market & Sales Opportunity Forecasted to 2034
- DB-1303 (Duality Biologics)
- Product Profile
- Clinical Development
- Market & Sales Opportunity Forecasted to 2034
- Disitamab vedotin (Yantai Rongchang Pharmaceutical)
- Product Profile
- Clinical Development
- Market & Sales Opportunity Forecasted to 2034
- Sacituzumab Govitecan (Gilead Sciences)
- Product Profile
- Clinical Development
- Market & Sales Opportunity Forecasted to 2034
- Datopotamab deruxtecan (AstraZeneca/Daiichi Sankyo)
- Product Profile
- Clinical Development
- Market & Sales Opportunity Forecasted to 2034
- MRG002 (Miracogen)
- Product Profile
- Clinical Development
- Market & Sales Opportunity Forecasted to 2034
- Others
- Trastuzumab rezetecan (Jiangsu HengRui Medicine)
- Product Analysis
- Launch Timeline & Key Market Events for HER2-Low Metastatic Breast Cancer
- HER2-Low Metastatic Breast Cancer – Pricing & Reimbursement
- KOLs Insight (US, EU, JP)
- Unmet Needs
- Analysis of the progresses in terms of approvals & current pipeline;
- Impact on the treatment algorithm and product positioning
- Relevance of new targets/platforms/ therapies Uptake Share %
- Physicians Preferences for the new therapies
- Future Treatment Paradigm
- HER2-Low Metastatic Breast Cancer Competitor Landscape and Approvals Anticipated
- Future Treatment Algorithms and Competitor Positioning
- Key Data Summary for Emerging Treatment
- Annual Cost of Current & Emerging Treatment
- Late Phase Therapy Strategic Considerations in HER2-Low Metastatic Breast Cancer
- Market Outlook
- Key Findings
- Overview
- Country Specific Market Forecast to 2034
- Sales of Drugs to Treat HER2-Low Metastatic Breast Cancer in the Major Pharmaceutical Markets, 2020-2034
- Patient Share of HER2-Low Metastatic Breast Cancer by Therapies
- Market Forecast by Country
- United States
- United States Market for HER2-Low Metastatic Breast Cancer 2020-2034 (USD Million)
- United States Market for HER2-Low Metastatic Breast Cancer by Therapy 2020-2034 (USD Million)
- Germany
- Germany Market for HER2-Low Metastatic Breast Cancer 2020-2034 (USD Million)
- Germany Market for HER2-Low Metastatic Breast Cancer by Therapy 2020-2034 (USD Million)
- France
- France Market for HER2-Low Metastatic Breast Cancer 2020-2034 (USD Million)
- France Market for HER2-Low Metastatic Breast Cancer by Therapy 2020-2034 (USD Million)
- Italy • Italy Market for HER2-Low Metastatic Breast Cancer 2020-2034 (USD Million) • Italy Market for HER2-Low Metastatic Breast Cancer by Therapy 2020-2034 (USD Million)
- Spain
- Spain Market for HER2-Low Metastatic Breast Cancer 2020-2034 (USD Million)
- Spain Market for HER2-Low Metastatic Breast Cancer by Therapy 2020-2034 (USD Million)
- United Kingdom
- United Kingdom Market for HER2-Low Metastatic Breast Cancer 2020-2034 (USD Million)
- United Kingdom Market for HER2-Low Metastatic Breast Cancer by Therapy 2020-2034 (USD Million)
- Japan
- Japan Market for HER2-Low Metastatic Breast Cancer 2020-2034 (USD Million)
- Japan Market for HER2-Low Metastatic Breast Cancer by Therapy 2020-2034 (USD Million)
- United States
- Market Drivers and Constraints
- What Factors Are Driving the Market for HER2-Low Metastatic Breast Cancer?
- What Factors Are Constraining the Market for HER2-Low Metastatic Breast Cancer?
- Appendix
- Methodology