 |
市场调查报告书
商品编码
1842451
微生物鑑定:市场占有率分析、产业趋势、统计数据和成长预测(2025-2030 年)Microbial Identification - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030) |
||||||
※ 本网页内容可能与最新版本有所差异。详细情况请与我们联繫。
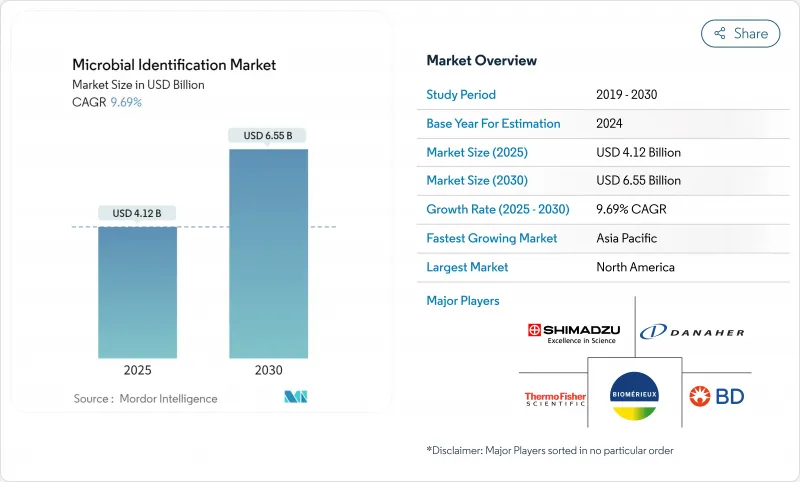
预计微生物鑑定市场在 2025 年的价值将达到 41.2 亿美元,到 2030 年将达到 65.5 亿美元,复合年增长率为 9.69%。
从基于培养的检测方法向分子平台的转变、抗菌素抗药性监测的加强以及对快速週转的期望是维持发展势头的关键因素。供应商正在扩展其技术组合,监管机构正在明确核准途径,医疗保健系统正在投资即时数据整合。同时,人员短缺和高资本要求阻碍了资源受限环境的采用。随着人工智慧工具扩展病原体库以及新兴经济体食品安全法规的收紧,长期成长前景依然强劲。
全球微生物鑑定市场趋势与洞察
MALDI-TOF MS 在常规诊断中的快速应用
使用每小时最多可处理 600 个样本的高通量 MALDI-TOF 平台,实验室现在可以在几分钟内(而非几小时)完成物种级别的鑑定,并以更低的试剂成本获得与 16S rRNA 测序精度相当的结果。凭藉涵盖 4,300 多个物种的扩展参考资料资料库,同一仪器可以支援食品、药品和临床工作流程。 2025 年 6 月,美国食品药物管理局将这些系统归类为具有特殊控制措施的 II 类系统,为製造商提供更清晰、更快捷的清关流程,同时保持安全标准。
抗菌素抗药性监测计画的成长
美国每年有超过280万人感染抗药性细菌,3.5万人死亡,全基因组定序已纳入监测网路。中国国家CHINET计画报告称,到2021年,分离出的肠桿菌菌株中10%对卡巴培南类抗生素抗药性,这凸显了全球对快速鑑定细菌的压力。及时的细菌分析可以帮助药师制定有效的治疗方案,并减少住院时间。
昂贵的设备和维护成本
先进的MALDI-TOF系统资本支出超过20万美元,服务合约每年使购买价格增加10-15%,限制了中型医院的采用。 2024年通过的新《临床实验室改进法案》绩效目标要求更严格的西格玛指标,这可能会迫使小型实验室提前升级或更换设备。
其他驱动因素和限制因素分析
- 新兴国家食品安全法规日益加强
- 人工智慧驱动的频谱库集成
- 熟练质谱技术人员短缺
細項分析
由于实验室每次检测都需要大量试剂和培养基,到2024年,耗材将占总收入的47.15%,这将使微生物鑑定市场在经常性现金流方面保持韧性。软体和服务虽然规模较小,但随着实验室升级到可自动进行资料移动和分析的云端实验室资讯系统,其复合年增长率达到11.78%,成为成长最快的细分市场。融合机器人技术和人工智慧的下一代「暗实验室」展示了软体层面如何在提高吞吐量的同时缓解劳动力短缺。
这一转变也凸显了分析仪錶板向订阅授权模式的广泛趋势,这将为供应商提供可预测的利润,并为用户带来快速的投资回报。随着品管法规的收紧,能够即时记录仪器性能并标记偏差的云端託管平台正变得越来越重要。预计到2030年,这类软体的采用率将维持两位数的成长,从而巩固数位化流程作为整个微生物鑑定市场核心竞争优势的地位。
由于无与伦比的快速结果获取速度、低廉的单次检测成本以及不断扩展的生物库,MALDI-TOF MS 将在 2024 年保持 57.50% 的收入份额。儘管 MALDI-TOF 平台的微生物鑑定市场规模仍在扩大,但随着北美和欧洲的采用率不断提高,其成长速度正在放缓。相较之下,随着多重检测板和照护现场模式在基层医疗诊所的普及,PCR 和即时PCR将以 12.73% 的复合年增长率成为成长最快的技术。 2024 年,四款领先的综合 PCR 分析仪获得 FDA 批准,彰显了监管的强劲势头。
混合工作流程正在兴起,实验室最初采用MALDI-TOF进行筛检,然后转向PCR和抗药性基因定序,兼顾广度和深度。跨平台资料融合正在催生新的耗材和服务组合,使製造商能够保护市场份额,同时利用互补分子检测带来的收入成长。
区域分析
2024年,北美仍将是最大的收入贡献地区,占全球整体的39.56%。这得益于其资金充足的医疗保健体系、可报销的快速检测费用以及强大的抗菌药物抗药性监测津贴。美国各地的实验室正在利用美国疾病管制与预防中心(CDC)的抗菌药物抗药性实验室网络,并采用联网识别平台,将即时数据输入国家资讯中心。加拿大也面临类似的发展趋势,但面临技术人员短缺的问题,导致较小州的设备采用速度放缓。
亚太地区预计将以11.45%的复合年增长率成长,这得益于中国和印度公立医院的扩张、东协倡议带来的品质标准协调,以及蓬勃发展的区域生物製造基地。 CHINET计画的多中心资料集展现了该地区资料的成熟度,以及由此带来的生物分析在指南抗生素处方方面的加速发展。各国政府也正在为地方疾病管制中心的设备采购提供补贴,以扩大农村地区的药物可近性。
欧洲保持了温和成长,因为严格的体外诊断监管期限促使实验室提前检验平台,从而确保了对相容套件的稳定需求。英国的ESPAUR报告指出,自2019年以来,抗菌药物抗药性负担增加了3.5%,因此将快速识别列入了政策议程。虽然英国脱欧导致的海关变化可能导致供应链延迟,但欧洲大陆的采购框架在很大程度上保护了最终用户免受短缺的影响。
中东和非洲地区尚处于采用的早期阶段,但受益于海湾国家对三级医疗机构和捐助者资助的水病原体计划的投资。随着巴西和墨西哥与主要贸易伙伴协调出口要求并扩大农业实验室的可近性,拉丁美洲的食品安全检测量正在增加。
其他福利:
- Excel 格式的市场预测 (ME) 表
- 3个月的分析师支持
目录
第一章 引言
- 研究假设和市场定义
- 调查范围
第二章调查方法
第三章执行摘要
第四章 市场状况
- 市场概况
- 市场驱动因素
- MALDI-TOF MS 在常规诊断中的快速应用
- 抗菌素抗药性(AMR)监测计画的成长
- 新兴国家食品安全法规日益加强
- 人工智慧频谱库整合(漏报)
- 分散式 POCT 微生物辨识系统的扩展(报告不足)
- 市场限制
- 设备和维护成本高
- 熟练质谱仪技术人员短缺
- 环境分离株缺乏标准化(漏报)
- 云端基础的身份平台中未被充分通报的网路安全风险
- 价值/供应链分析
- 监管状况
- 技术展望
- 五力分析
- 新进入者的威胁
- 供应商的议价能力
- 买方的议价能力
- 替代品的威胁
- 竞争对手之间的竞争
第五章市场规模及成长预测
- 按产品/服务
- 装置
- 耗材
- 软体和服务
- 依技术
- MALDI-TOF MS
- PCR和即时PCR
- 定序(NGS、Sanger)
- 其他(生物化学、显微镜等)
- 按最终用户
- 医院和临床实验室
- 製药和生物技术公司
- 食品饮料检验实验室
- 环境和工业测试实验室
- 按用途
- 临床诊断
- 製药製造品质控制
- 食品安全/品质
- 环境监测
- 按地区
- 北美洲
- 美国
- 加拿大
- 墨西哥
- 欧洲
- 德国
- 英国
- 法国
- 义大利
- 西班牙
- 其他欧洲国家
- 亚太地区
- 中国
- 印度
- 日本
- 韩国
- 澳洲
- 其他亚太地区
- 南美洲
- 巴西
- 阿根廷
- 其他南美
- 中东和非洲
- GCC
- 南非
- 其他中东和非洲地区
- 北美洲
第六章 竞争态势
- 市场集中度
- 市占率分析
- 公司简介
- bioMerieux SA
- Bruker Corporation
- Becton, Dickinson and Company
- Thermo Fisher Scientific Inc.
- Danaher Corporation(Beckman Coulter)
- Shimadzu Corporation
- Charles River Laboratories
- Biolog Inc.
- Qiagen NV
- Merck KGaA(MilliporeSigma)
- Liofilchem Srl
- bioNote Inc.
- MIDI Labs
- Eppendorf AG
- Hologic Inc.
- Roche Diagnostics
- Siemens Healthineers
- Revvity(PerkinElmer)
- Abbott Laboratories
- Agilent Technologies
第七章 市场机会与未来展望
The microbial identification market was valued at USD 4.12 billion in 2025 and is forecast to reach USD 6.55 billion by 2030, advancing at a 9.69% CAGR.

The transition from culture-based assays to molecular platforms, intensified antimicrobial-resistance surveillance, and quicker turnaround expectations are the key forces sustaining momentum. Vendors are broadening technology portfolios, regulators are clarifying approval pathways, and healthcare systems are investing in real-time data integration. At the same time, staffing shortages and high capital requirements temper adoption in resource-constrained settings. Long-term growth prospects remain strong as artificial-intelligence tools extend pathogen libraries and as food-safety rules tighten across emerging economies.
Global Microbial Identification Market Trends and Insights
Rapid Adoption of MALDI-TOF MS in Routine Diagnostics
Laboratories now generate species-level identification within minutes rather than hours by using high-throughput MALDI-TOF platforms that process up to 600 samples per hour, matching the accuracy of 16S rRNA sequencing at lower reagent cost. Expanded reference databases covering more than 4,300 species enable the same instrument to support food, pharmaceutical, and clinical workflows. The United States Food and Drug Administration placed these systems in Class II with special controls in June 2025, giving manufacturers a clearer, faster clearance route while preserving safety standards .
Growth of Antimicrobial-Resistance Surveillance Programs
More than 2.8 million AMR infections occurred annually in the United States, resulting in 35,000 deaths, which prompted whole-genome sequencing adoption across surveillance networks. China's national CHINET program reported carbapenem resistance in 10% of Enterobacter isolates by 2021, highlighting convergent global pressure for rapid identification. Timely organism profiling helps pharmacists tailor effective therapy and shorten hospital stays.
High Instrument and Maintenance Costs
Capital expenditure for an advanced MALDI-TOF system can exceed USD 200,000, while service contracts add 10-15% of purchase price each year, restricting uptake in mid-tier hospitals. New Clinical Laboratory Improvement Amendments performance goals adopted in 2024 require tighter sigma metrics, which may oblige smaller labs to upgrade or replace equipment sooner than planned.
Other drivers and restraints analyzed in the detailed report include:
- Rising Food-Safety Regulations in Emerging Economies
- Integration of AI-Powered Spectral Libraries
- Shortage of Skilled Mass-Spectrometry Technicians
For complete list of drivers and restraints, kindly check the Table Of Contents.
Segment Analysis
Consumables generated 47.15% of 2024 revenues as labs relied on high-volume reagents and media needed for every run, giving the microbial identification market recurring cash flow resilience. Software and services, though smaller, are growing the fastest at 11.78% CAGR as laboratories upgrade to cloud laboratory-information systems that automate data movement and analytics. Next-generation "dark labs" showcasing robotics and AI illustrate how software layers mitigate staffing gaps while boosting throughput .
The shift also highlights a broader move toward subscription licensing for analytics dashboards, offering predictable margins to vendors and quicker payback for users. As quality-control regulations tighten, cloud-hosted platforms that log instrument performance and flag deviations in real time are becoming critical. This software uptake is expected to maintain double-digit growth through 2030, cementing digital processes as a core competitive differentiator across the microbial identification market.
MALDI-TOF MS retained a 57.50% revenue share in 2024 on the strength of unmatched speed-to-result, low per-test cost, and a continuously expanding organism library. The microbial identification market size for MALDI-TOF platforms is still expanding, yet growth is moderating as penetration rises in North America and Europe. PCR and real-time PCR, by contrast, will post the sharpest 12.73% CAGR through 2030 as multiplex panels and point-of-care formats reach primary-care clinics. Four separate FDA clearances for a flagship syndromic PCR analyzer in 2024 illustrate regulatory momentum.
Hybrid workflows are emerging in which laboratories first screen with MALDI-TOF, then reflex to PCR or sequencing for resistance genes, combining breadth with depth. Cross-platform data convergence is spurring new consumable and service bundles, allowing manufacturers to defend share while tapping incremental revenue from complementary molecular assays.
The Microbial Identification Market Report Segments by Products and Services (Instruments, Consumables and More), by Technology (MALDI-TOF, MSPCR & Real-Time and More ), by End User (Hospitals & Clinical Laboratories and More), by Application (Clinical Diagnostics, Pharmaceutical Manufacturing QC and More) and Geography (North America and More). The Market Forecasts are Provided in Terms of Value (USD).
Geography Analysis
North America remained the largest revenue contributor in 2024, claiming 39.56% of global spend, reflecting well-funded healthcare systems, reimbursed rapid tests, and robust AMR surveillance grants. Laboratories across the United States leverage the CDC's Antimicrobial Resistance Laboratory Network to adopt connected identification platforms that feed real-time data into national dashboards. Canada follows similar trajectories but faces greater technician shortages, delaying instrument rollouts in smaller provinces.
Asia-Pacific, forecast to rise at 11.45% CAGR, is propelled by public hospital expansion in China and India, harmonized quality standards under ASEAN initiatives, and a vibrant local biomanufacturing base. The CHINET program's multicenter datasets illustrate the region's data maturity and the resulting push for faster organism profiling to guide antibiotic formularies. Governments are also subsidizing instrument purchases for provincial disease-control centers, widening rural access.
Europe maintains moderate growth as stringent In-Vitro Diagnostic Regulation deadlines drive labs to validate platforms earlier than scheduled, ensuring steady demand for compliant kits. The United Kingdom's ESPAUR report cites a 3.5% rise in AMR burden since 2019, keeping rapid identification on policy agendas. Brexit customs changes create occasional supply chain delays, yet continental procurement frameworks largely shield end users from shortages.
The Middle East and Africa region is at an earlier adoption stage but benefits from Gulf state investment in tertiary care facilities and from donor-funded water-pathogen projects. Latin America sees rising food-safety testing volumes as Brazil and Mexico align export requirements with major trade partners, boosting uptake among agro-industry labs.
- bioMerieux
- Bruker
- Beckton Dickinson
- Thermo Fisher Scientific
- Danaher
- Shimadzu
- Charles River
- Biolog
- QIAGEN
- Merck KGaA (MilliporeSigma)
- Liofilchem Srl
- bioNote Inc.
- MIDI Labs
- Eppendorf
- Hologic
- Roche
- Siemens Healthineers
- Revvity (PerkinElmer)
- Abbott Laboratories
- Agilent Technologies
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 Introduction
- 1.1 Study Assumptions & Market Definition
- 1.2 Scope of the Study
2 Research Methodology
3 Executive Summary
4 Market Landscape
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Rapid adoption of MALDI-TOF MS in routine diagnostics
- 4.2.2 Growth of antimicrobial-resistance (AMR) surveillance programs
- 4.2.3 Rising food-safety regulations in emerging economies
- 4.2.4 Integration of AI-powered spectral libraries (under-reported)
- 4.2.5 Expansion of decentralized POCT microbial ID systems (under-reported)
- 4.3 Market Restraints
- 4.3.1 High instrument & maintenance costs
- 4.3.2 Shortage of skilled mass-spectrometry technicians
- 4.3.3 Lack of standardization for environmental isolates (under-reported)
- 4.3.4 Cyber-security risks in cloud-based ID platforms (under-reported)
- 4.4 Value/ Supply-Chain Analysis
- 4.5 Regulatory Landscape
- 4.6 Technological Outlook
- 4.7 Porter's Five Forces
- 4.7.1 Threat of New Entrants
- 4.7.2 Bargaining Power of Suppliers
- 4.7.3 Bargaining Power of Buyers
- 4.7.4 Threat of Substitutes
- 4.7.5 Competitive Rivalry
5 Market Size & Growth Forecasts (Value, USD)
- 5.1 By Product & Service
- 5.1.1 Instruments
- 5.1.2 Consumables
- 5.1.3 Software & Services
- 5.2 By Technology
- 5.2.1 MALDI-TOF MS
- 5.2.2 PCR & Real-time PCR
- 5.2.3 Sequencing (NGS, Sanger)
- 5.2.4 Others (Biochemical, Microscopy, etc.)
- 5.3 By End-User
- 5.3.1 Hospitals & Clinical Laboratories
- 5.3.2 Pharmaceutical & Biotechnology Companies
- 5.3.3 Food & Beverage Testing Labs
- 5.3.4 Environmental & Industrial Labs
- 5.4 By Application
- 5.4.1 Clinical Diagnostics
- 5.4.2 Pharmaceutical Manufacturing QC
- 5.4.3 Food Safety & Quality
- 5.4.4 Environmental Monitoring
- 5.5 By Geography
- 5.5.1 North America
- 5.5.1.1 United States
- 5.5.1.2 Canada
- 5.5.1.3 Mexico
- 5.5.2 Europe
- 5.5.2.1 Germany
- 5.5.2.2 United Kingdom
- 5.5.2.3 France
- 5.5.2.4 Italy
- 5.5.2.5 Spain
- 5.5.2.6 Rest of Europe
- 5.5.3 Asia-Pacific
- 5.5.3.1 China
- 5.5.3.2 India
- 5.5.3.3 Japan
- 5.5.3.4 South Korea
- 5.5.3.5 Australia
- 5.5.3.6 Rest of Asia-Pacific
- 5.5.4 South America
- 5.5.4.1 Brazil
- 5.5.4.2 Argentina
- 5.5.4.3 Rest of South America
- 5.5.5 Middle East and Africa
- 5.5.5.1 GCC
- 5.5.5.2 South Africa
- 5.5.5.3 Rest of Middle East and Africa
- 5.5.1 North America
6 Competitive Landscape
- 6.1 Market Concentration
- 6.2 Market Share Analysis
- 6.3 Company Profiles (includes Global level Overview, Market level overview, Core Segments, Financials as available, Strategic Information, Market Rank/Share, Products & Services, Recent Developments)
- 6.3.1 bioMerieux SA
- 6.3.2 Bruker Corporation
- 6.3.3 Becton, Dickinson and Company
- 6.3.4 Thermo Fisher Scientific Inc.
- 6.3.5 Danaher Corporation (Beckman Coulter)
- 6.3.6 Shimadzu Corporation
- 6.3.7 Charles River Laboratories
- 6.3.8 Biolog Inc.
- 6.3.9 Qiagen N.V.
- 6.3.10 Merck KGaA (MilliporeSigma)
- 6.3.11 Liofilchem Srl
- 6.3.12 bioNote Inc.
- 6.3.13 MIDI Labs
- 6.3.14 Eppendorf AG
- 6.3.15 Hologic Inc.
- 6.3.16 Roche Diagnostics
- 6.3.17 Siemens Healthineers
- 6.3.18 Revvity (PerkinElmer)
- 6.3.19 Abbott Laboratories
- 6.3.20 Agilent Technologies
7 Market Opportunities & Future Outlook
- 7.1 White-space & Unmet-Need Assessment










