 |
市场调查报告书
商品编码
1762540
细胞系表征和细胞系开发市场:行业趋势和全球预测 - 依细胞系/表达系统来源、细胞系应用、公司规模和主要地区划分Cell Line Characterization and Cell Line Development Market: Industry Trends and Global Forecasts - Distribution by Source of Cell Line / Expression System, Application of Cell Line, Company Size and Key Geographical Regions |
||||||
细胞系表征与细胞系开发市场:概览
预计到2035年,全球细胞系表征和细胞系开发市场规模将从目前的22.9亿美元成长至83.8亿美元,在预测期内,年复合成长率将达到12.5%。
市场区隔包括以下参数的市场规模和机会分析:
细胞系/表现系统来源
- 哺乳动物
- 微生物
- 昆虫
- 其他
细胞的应用系
- 研发
- 生物製造
公司规模
- 超大型
- 大型
- 中型
- 小型
主要地区
- 北美
- 欧洲
- 亚太地区
- 世界其他地区
细胞系表征与细胞系开发市场:成长与趋势
多年来,细胞系(培养的细胞群)对生物製药产业非常重要。它们是筛选潜在候选药物、开发生物製剂、研究慢性疾病生物学、评估药物毒性的宝贵工具。此外,可以对已开发的细胞系进行表征和鑑定,以确定其来源和历史,并识别其关键特性和功能。虽然目前使用的细胞系大多源自人类、小鼠和大鼠,但也有一些源自其他哺乳动物和非哺乳动物物种,拓宽了它们在研发中的应用。
此外,值得注意的是,随着时间的推移,基因组编辑技术的进步为细胞系开发开闢了新的途径。然而,细胞系开发和表征在技术上具有挑战性,并且在经济上要求很高。因此,药物开发公司越来越依赖拥有先进技术、基础设施和专业知识的合约服务提供者。近年来,涌现出许多高素质的合约研究组织(CRO)和合约製造组织(CMO),它们为药物开发者提供帮助,并加快细胞系开发和生物製造。
细胞系表征与细胞系开发市场:关键洞察
本报告深入探讨了细胞系表征和细胞系开发市场的现状,并识别了行业内的潜在成长机会。报告的主要调查结果包括:
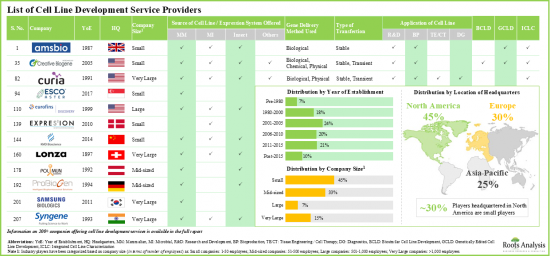
- 目前,超过200 家公司(包括新创公司和成熟公司)声称拥有提供细胞系开发服务的必要能力,其中大多数公司位于北美。
- 利害关係人有能力开发从各种来源获得的细胞系,以支持基于细胞的研发运作并促进生物治疗药物的生产。
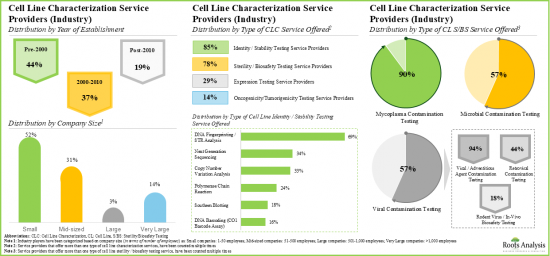
- 超过 140 家服务提供者(包括工业和非工业)声称提供细胞系表征服务。其中相当一部分公司提供基因分型服务,以确定细胞系的身份和稳定性。
- 大多数细胞系表征服务提供者都是2000年后成立的中小型企业。
- 为了满足日益成长的利用细胞系开发的新型生物製剂的需求,服务提供者提升其技术专长并增加新功能以扩充其服务组合。
- 老牌公司和新进业者都在建立策略伙伴关係,其中授权协议正成为最主要的合作模式。
- 为了满足日益成长的细胞系需求,各公司大力投资以扩大其设施和能力。这一趋势在美国和中国尤为突出。
- 识别进步的驱动因素和阻碍研究进展的障碍有助于改善策略规划并实现高效运作。
- 长期来看,细胞系开发服务市场预计将成长 12.5%,并且在不同细胞系来源、应用领域和地区,市场机会均呈现多元化。
- 随着製药公司和研究人员持续外包细胞系表征活动,预计未来十年细胞系表征市场将以每年超过 10%的速度成长。
细胞系表征与细胞系开发市场:关键细分市场
根据细胞系/表现系统的来源,市场细分为哺乳动物、微生物、昆虫和其他。目前,哺乳动物细胞系在全球细胞系表征和细胞系开发市场中占据最大占有率。预计未来几年这一趋势将保持不变。
依细胞系应用,市场细分为研发和生物製造。目前,生物製造领域在全球细胞系表征和细胞系开发市场中占比最高。此外,由于实验研究对高品质、可靠的细胞系的需求日益成长,预计未来细胞系在研发领域的应用机会将持续扩大。
依公司规模,市场细分为超大型、大型、中型和小型。目前,中型公司在全球细胞系表征和细胞系开发市场中占据最大占有率。此外,预计未来该领域的年复合成长率将更高。
依主要地区,市场细分为北美、欧洲、亚太地区和世界其他地区。目前,北美在全球细胞系表征和细胞系开发市场中占据主导地位,占据最大的收入占有率。此外,亚太市场未来可望实现更高的年复合成长率。
进入细胞系表征和细胞系开发市场的公司范例
- ATUM
- ATZ Labs(Life Technologies 的子公司)
- Avance Biosciences
- BioReliance(已被 Sigma-Aldrich 收购)
- Biovian
- Celonic Group
- Charles River Laboratories
- ChemPartner
- Cleancells
- Creative Biogene
- Curia
- Eurofins BioPharma Product Testing
- FUJIFILM Diosynth Biotechnologies
- Hylabs
- KBI Biopharma
- Kemp Proteins
- KMD Bioscience
- Livogen Pharmed
- Lonza
- Molecular Diagnostic Services
- Mycenax Biotech
- ProBioGen
- Samsung BioLogics
- Sartorius
- SGS Life Sciences
- Syngene International
- Texcell
- TFBS Bioscience
- Thermo Fisher Scientific
- WuXi Advanced Therapies
- WuXi Biologics
目录
第1章 简介
第2章 研究方法
第3章 经济及其他专案特定考量
第4章 执行摘要
第5章 简介
- 章节概述
- 细胞培养概述
- 细胞系表征概述
- 细胞系的应用
- 与细胞系相关的主要问题
- 细胞系业务外包的必要性
- 结论
第6章 细胞系开发服务提供者:市场格局
- 章节概述
- 细胞系开发服务提供者:市场格局
第7章 细胞系开发服务提供者:公司竞争力分析
- 章节概述
- 假设/关键参数
- 研究方法
- 细胞系开发服务提供者:公司竞争力分析
第8章 细胞系开发服务提供者公司简介
- 章节概述
- 主要服务公司详细简介提供者
- ATUM
- Curia
- Fujifilm Diosynth Biotechnologies
- Lonza
- Syngene International
- WuXi Biologics
- 其他服务提供者的公司简介
- Biovian
- Celonic
- ChemPartner
- Creative Biogene
- KBI Biopharma
- Kemp Proteins
- KMD Bioscience
- Mycenax Biotech
- ProBioGen
- Thermo Fisher Scientific
第9章 细胞系表征服务提供者:市场市场模式
- 章节概述
- 细胞系表征服务提供者:市场格局
第10章 细胞系表征服务提供者:公司竞争力分析
- 章节概述
- 假设/关键参数
- 研究方法
- 细胞系表征服务提供者:公司竞争力分析
第11章 细胞系表征服务提供者公司简介
- 章节概述
- 主要服务前景投标人的详细公司简介
- Charles River Laboratories
- Eurofins BioPharma Product Testing
- Livogen Pharmed
- Molecular Diagnostic Services
- Sartorius
- TFBS Bioscience
- 其他服务提供者的公司简介
- ATZ Labs(Subsidiary of Life Technologies)
- Avance Biosciences
- BioReliance
- Clean Cells
- hylabs
- Samsung Biologics
- SGS Life Sciences
- Texcell
- WuXi Advanced Therapies
章节12 伙伴关係与合作
- 章节概述
- 合作模式
- 细胞系开发与鑑定:伙伴关係与合作
第13章 近期扩展
- 章节概述
- 细胞系开发与鑑定:近期扩展列表
第14章 细胞系库
- 章节概述
- American Type Culture Collection (ATCC)
- Coriell Institute for Medical Research
- European Collection of Authenticated Cell Cultures (ECACC)
- Leibniz Institute DSMZ - German Collection of Microorganisms and Cell Cultures
- National Centre for Cell Science (NCCS)
第15章 监理建议及指引
- 章节概述
- 细胞系鑑定:主要监管机构
- 国际监理机构发布的指南
- 美国细胞系表征监管指南
- 欧洲细胞系表征调控指南
- 日本细胞系表征调控指南
- 结论
第16章 市场影响分析:驱动因素、限制因素、机会与挑战
第17章 全球细胞系开发服务市场
- 章节概述
- 假设与研究方法
- 全球细胞系开发服务市场,历史趋势(2019年至今)及预测(至2035年)
- 主要市场细分
第18章 细胞係依细胞系来源划分的细胞系开发服务市场
- 章节概述
- 关键假设与研究方法
- 以细胞系来源划分的细胞系开发服务市场
- 资料三角测量与验证
第19章 依细胞系应用划分的细胞系开发服务市场
- 章节概述
- 关键假设与研究方法
- 依细胞系应用划分的细胞系开发服务市场
- 资料三角测量与验证
第20章 依公司规模划分的细胞系开发服务市场
- 章节概述
- 关键假设与研究方法
- 依公司规模划分的细胞系开发服务市场
- 资料三角测量与验证
第21章 按关键地区
- 章节概述
- 关键假设与研究方法
- 依主要地区划分的细胞系开发服务市场
- 资料三角测量与验证
第22章 全球细胞系表征服务市场
- 章节概述
- 关键假设与研究方法
- 全球细胞系表征服务市场,历史趋势(2019年起)与预测(至2035年)
- 主要市场细分
第23章 依细胞系来源划分的细胞系表征服务市场
- 章节概述
- 关键假设与研究方法
- 依细胞系来源划分的细胞系表征服务市场
- 资料三角测量与验证
章节第24章 细胞系表征服务市场(依细胞系应用)
- 章节概述
- 关键假设与研究方法
- 细胞系表征服务市场(依细胞系应用)
- 资料三角测量与验证
第25章 细胞系表征服务市场(依公司规模)
- 章节概述
- 关键假设与研究方法
- 细胞系表征服务市场(依公司规模)
- 资料三角测量与验证
第26章 细胞系表征服务市场(依主要地区)
- 章节概述
- 关键假设与研究方法
- 细胞系表征服务市场(依主要地区)
- 资料三角测量与验证
第27章 结论
第28章 高层洞察
第29章 附录1:表格资料
第30章 附录2:公司与组织清单
第31章 附录3:合作伙伴关係及合作详情
CELL LINE CHARACTERIZATION AND CELL LINE DEVELOPMENT MARKET: OVERVIEW
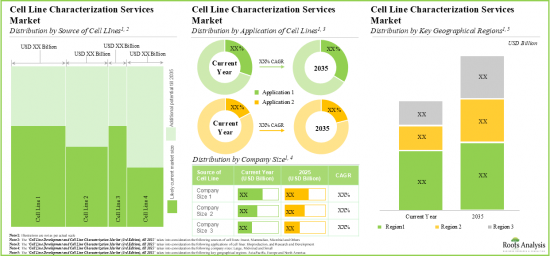
As per Roots Analysis, the global cell line characterization and cell line development market is estimated to grow from USD 2.29 billion in the current year to USD 8.38 billion by 2035, at a CAGR of 12.5% during the forecast period, till 2035.
The market sizing and opportunity analysis has been segmented across the following parameters:
Source of Cell Line / Expression System
- Mammalian
- Microbial
- Insect
- Others
Application of Cell Line
- Research and Development
- Biomanufacturing
Company Size
- Very Large
- Large
- Mid-sized
- Small
Key Geographical Regions
- North America
- Europe
- Asia Pacific
- Rest of the World
CELL LINE CHARACTERIZATION AND CELL LINE DEVELOPMENT MARKET: GROWTH AND TRENDS
Over the years, cell lines (cultured populations of cells) have become a crucial part of the biopharmaceutical industry. They are a valuable tool for screening potential drug candidates, developing biologics, studying biology of chronic diseases and assessing toxicity of drugs. Moreover, the developed cell lines can be characterized and authenticated to determine their origin / history and identify key characteristics and functionality. While most cell lines in use today are derived from humans, mice, and rats, some also originate from other mammalian and non-mammalian species, broadening their applicability across research and development.

Further, it is worth highlighting that over time the increasing advancements in genome editing technology have opened new avenues for cell line development. Despite this, the development and characterization of cell lines is both technically challenging and financially demanding; as a result, drug developers are becoming increasingly dependent on contract service providers for their advanced technologies, infrastructure and expertise. Recent years have witnessed the emergence of a large number of highly qualified contract research organizations (CROs) and contract manufacturing organizations (CMOs) that assist drug developers and strive to expedite cell line development and biomanufacturing.
CELL LINE CHARACTERIZATION AND CELL LINE DEVELOPMENT MARKET: KEY INSIGHTS
The report delves into the current state of the cell line characterization and cell line development market and identifies potential growth opportunities within industry. Some key findings from the report include:
- Presently, over 200 players (established as well as startups) claim to have the necessary capabilities to offer cell line development services; majority of these firms are based in North America.
- Stakeholders have the capability to develop cell lines obtained from different sources that support cell-based research and development operations and facilitate production of biotherapeutics.
- Over 140 service providers (industry and non-industry) claim to offer cell line characterization services; a sizeable proportion of these players offer genotyping services for accessing the identity and stability of cell lines.
- Majority of the cell line characterization service providers are small and mid-sized firms, established post-2000; notably, around 10% of the players offer all types of characterization services

- In order to cater to the rising demand of novel biologics (developed using cell lines), service providers are upgrading their technical expertise and adding new competencies to augment their service portfolios.
- Both well-established players and new entrants have forged strategic partnerships; licensing agreements emerged as the most prominent partnership model.
- To keep pace with the growing demand for cell lines, companies have made significant investments to expand their facilities and capacities; this trend is most pronounced in the US and China.
- Identifying the driving factors (that fuel advancements) as well as barriers (that slow down the research progress) helps improve strategic planning and results in efficient operations.
- In the long term, cell line development services market is expected to witness growth of 12.5%; the opportunity is likely to be well distributed across different sources of cell lines, application areas and geographies.

- As pharmaceutical companies and researchers continue outsourcing cell line characterization operations, we anticipate cell line characterization market to grow at an annualized rate of more than 10%, over the next decade.
CELL LINE CHARACTERIZATION AND CELL LINE DEVELOPMENT MARKET: KEY SEGMENTS
Mammalian Cell Line Segment holds the Largest Share of the Global Cell Line Characterization and Cell Line Development Market
Based on the source of cell line / expression system, the market is segmented into mammalian, microbial, insect, and others. At present, the mammalian cell line segment holds the maximum share of the global cell line characterization. This trend is likely to remain the same in the coming years.
By Application of Cell Line, Biomanufacturing is the Fastest Growing Segment of the Global Cell Line Characterization and Cell Line Development Market
Based on the application of cell line, the market is segmented into research and development and biomanufacturing. Currently, the biomanufacturing segment captures the highest proportion of the global cell line characterization and cell line development market. Further due to the growing need for high quality and authentic cell lines for experimental research, the opportunity for cell line application in research and development is anticipated to grow in the future.
Mid-sized Companies Segment Occupy the Largest Share of the Global Cell Line Characterization and Cell Line Development Market
Based on the company size, the market is segmented into very large, large, mid-sized, and small. At present, the mid-sized segment holds the maximum share of the global cell line characterization and cell line development market. Further this segment is expected to grow at a higher CAGR in the coming future.
North America Accounts for the Largest Share of the Market
Based on key geographical regions, the market is segmented into North America, Europe, Asia-Pacific and Rest of the World. Currently, North America dominates the global cell line characterization and cell line development market and accounts for the largest revenue share. Further, the market Asia-Pacific is likely to grow at a higher CAGR in the coming future.
Example Players in the Cell Line Characterization and Cell Line Development Market
- ATUM
- ATZ Labs (a subsidiary of Life Technologies)
- Avance Biosciences
- BioReliance (acquired by Sigma-Aldrich)
- Biovian
- Celonic Group
- Charles River Laboratories
- ChemPartner
- Cleancells
- Creative Biogene
- Curia
- Eurofins BioPharma Product Testing
- FUJIFILM Diosynth Biotechnologies
- Hylabs
- KBI Biopharma
- Kemp Proteins
- KMD Bioscience
- Livogen Pharmed
- Lonza
- Molecular Diagnostic Services
- Mycenax Biotech
- ProBioGen
- Samsung BioLogics
- Sartorius
- SGS Life Sciences
- Syngene International
- Texcell
- TFBS Bioscience
- Thermo Fisher Scientific
- WuXi Advanced Therapies
- WuXi Biologics
CELL LINE CHARACTERIZATION AND CELL LINE DEVELOPMENT MARKET: RESEARCH COVERAGE
- Market Sizing and Opportunity Analysis: The report features an in-depth analysis of the global cell line characterization and cell line development market, focusing on key market segments, including [A] source of cell line / expression system, [B] application of cell line, [C] company size and [D] key geographical regions.
- Cell Line Development Service Providers Market Landscape: A comprehensive evaluation of cell line development service providers, based on several relevant parameters, such as [A] year of establishment, [B] company size, [C] location of headquarters, [D] source of cell lines / expression systems offered, [E] gene delivery method used, [F] type of transfection, [G] availability of serum free / animal component free culturing capability, [H] type of cells offered, [I] application of cell lines, [J] additional cell line related services offered, [K] type of cell banking, [L] availability of integrated cell line characterization, [M] technology platform utilized, [N] monoclonality procedure used, [O] availability of biosimilar cell line development and [P] gene editing cell line development services.
- Cell Line Development Service Providers Company Competitiveness Analysis: A comprehensive competitive analysis of cell line development service providers, examining factors, such as [A] supplier strength and [B] service strength.
- Company Profiles of Cell Line Development Service Providers: In-depth profiles of key players offering cell line development services, focusing on [A] overview of the company, [B] financial information (if available), [C] service portfolio, [D] recent developments and an informed future outlook. Additionally, in-depth profiles of some other prominent players focusing on [E] overview of the company and [F] cell line development service portfolio.
- Cell Line Characterization Service Providers Market Landscape: A comprehensive evaluation of the companies / organizations providing cell line characterization services, based on several relevant parameters, such as [A] year of establishment, [B] company size, [C] location of headquarters, [D] source of cell lines and expression systems offered, [E] type of cell line characterization services offered, [F] type of cell identity testing / cell stability testing services offered, [G] type of sterility / biosafety testing services offered, [H] availability of additional cell line related services, [I] regulatory accreditations / certifications and [J] overall turnaround time. Additional information on [K] number of STR loci amplified, [L] type of genotyping kit used, and [M] service fee charged for non-industry players.
- Cell Line Characterization Service Providers Company Competitiveness Analysis: A comprehensive competitive analysis of cell line characterization service providers, examining factors, such as [A] supplier strength and [B] service strength.
- Company Profiles of Cell Line Characterization Service Providers: In-depth profiles of key players offering cell line characterization services, focusing on [A] overview of the company, [B] financial information (if available), [C] cell line characterization service portfolio, [D] recent developments and an informed future outlook. Additionally, in-depth profiles of some other prominent players focusing on [E] overview of the company and [F] cell line characterization service portfolio.
- Partnerships and Collaborations: An insightful analysis of the deals inked by stakeholders in this domain, based on several parameters, such as [A] year of partnership, [B] type of partnership, [C] type of cells involved, [D] therapeutic area, [E] type of partner, [F] most active players (in terms of the number of partnerships signed) and [G] regional distribution of the companies involved in these agreements.
- Recent Expansions: In-depth analysis of the various expansion initiatives undertaken by various cell line development and cell line characterization service providers, based on several parameters, such as [A] year of expansion, [B] type of expansion, [C] location of headquarters, [D] location of expanded facility, [E] area of expanded facility, [F] purpose of expansion, [G] most active players (in terms of number of recent expansions) and [H] geographical distribution.
- Cell Line Repositories: In-depth profiles of the biorepositories across the globe that play an important role in developing cell lines and have also undertaken initiatives to limit the use of contaminated and / or misidentified cell lines, focusing on [A] overview of the repository and [B] cell line characterization service portfolio.
- Regulatory Recommendations and Guidelines: A detailed discussion on the requirements established by various regulatory authorities, across different regions, related to characterization of cell lines. Additionally, a detailed discussion of the various guidelines that have been issued by these bodies related to the protocols that need to be followed while testing of cell lines. Further, a brief description of the historical overview and contributions of key institutes / organizations involved in this domain was represented.
- Market Impact Analysis: A thorough analysis of various factors, such as drivers, restraints, opportunities, and existing challenges that are likely to impact market growth.
KEY QUESTIONS ANSWERED IN THIS REPORT
- How many companies are currently engaged in this market?
- Which are the leading companies in this market?
- What factors are likely to influence the evolution of this market?
- What is the current and future market size?
- What is the CAGR of this market?
- How is the current and future market opportunity likely to be distributed across key market segments?
REASONS TO BUY THIS REPORT
- The report provides a comprehensive market analysis, offering detailed revenue projections of the overall market and its specific sub-segments. This information is valuable to both established market leaders and emerging entrants.
- Stakeholders can leverage the report to gain a deeper understanding of the competitive dynamics within the market. By analyzing the competitive landscape, businesses can make informed decisions to optimize their market positioning and develop effective go-to-market strategies.
- The report offers stakeholders a comprehensive overview of the market, including key drivers, barriers, opportunities, and challenges. This information empowers stakeholders to stay abreast of market trends and make data-driven decisions to capitalize on growth prospects.
ADDITIONAL BENEFITS
- Complimentary PPT Insights Packs
- Complimentary Excel Data Packs for all Analytical Modules in the Report
- 15% Free Content Customization
- Detailed Report Walkthrough Session with Research Team
- Free Updated report if the report is 6-12 months old or older
TABLE OF CONTENTS
1. PREFACE
- 1.1. Introduction
- 1.2. Project Objectives
- 1.3. Scope of the Report
- 1.4. Inclusions and Exclusions
- 1.5. Key Questions Answered
- 1.6. Chapter Outlines
2. RESEARCH METHODOLOGY
- 2.1. Chapter Overview
- 2.2. Research Assumptions
- 2.3. Project Methodology
- 2.4. Forecast Methodology
- 2.5. Robust Quality Control
- 2.6. Key Market Segmentations
- 2.7. Key Considerations
- 2.7.1. Demographics
- 2.7.2. Economic Factors
- 2.7.3. Government Regulations
- 2.7.4. Supply Chain
- 2.7.5. COVID Impact / Related Factors
- 2.7.6. Market Access
- 2.7.7. Healthcare Policies
- 2.7.8. Industry Consolidation
3. ECONOMIC AND OTHER PROJECT SPECIFIC CONSIDERATIONS
- 3.1. Chapter Overview
- 3.2. Market Dynamics
- 3.2.1. Time Period
- 3.2.1.1. Historical Trends
- 3.2.1.2. Current and Forecasted Estimates
- 3.2.2. Currency Coverage
- 3.2.2.1. Overview of Major Currencies Affecting the Market
- 3.2.2.2. Impact of Currency Fluctuations on the Industry
- 3.2.3. Foreign Exchange Impact
- 3.2.3.1. Evaluation of Foreign Exchange Rates and Their Impact on Market
- 3.2.3.2. Strategies for Mitigating Foreign Exchange Risk
- 3.2.4. Recession
- 3.2.4.1. Historical Analysis of Past Recessions and Lessons Learnt
- 3.2.4.2. Assessment of Current Economic Conditions and Potential Impact on the Market
- 3.2.5. Inflation
- 3.2.5.1. Measurement and Analysis of Inflationary Pressures in the Economy
- 3.2.5.2. Potential Impact of Inflation on the Market Evolution
- 3.2.1. Time Period
4. EXECUTIVE SUMMARY
5. INTRODUCTION
- 5.1. Chapter Overview
- 5.2. Overview of Cell Culture
- 5.2.1. Classification of Cell Cultures
- 5.2.1.1. Classification based on Origin
- 5.2.1.1.1. Primary Cell Cultures
- 5.2.1.1.2. Secondary Cell Cultures
- 5.2.1.2. Classification based on Growth Properties
- 5.2.1.2.1. Adherent Cell Cultures
- 5.2.1.2.2. Suspension Cell Cultures
- 5.2.1.1. Classification based on Origin
- 5.2.2. Classification of Cell Lines
- 5.2.2.1. Classification based on Lifespan of Cell Line
- 5.2.2.1.1. Finite Cell Lines
- 5.2.2.1.2. Continuous Cell Lines
- 5.2.2.2. Classification based on Type of Cell Line
- 5.2.2.2.1. Recombinant Cell Lines
- 5.2.2.2.2. Hybridoma Cell Lines
- 5.2.2.3. Classification based on Source of Cell Line
- 5.2.2.3.1. Mammalian Cell Lines
- 5.2.2.3.2. Non-Mammalian Cell lines
- 5.2.2.1. Classification based on Lifespan of Cell Line
- 5.2.1. Classification of Cell Cultures
- 5.3. Overview of Cell Line Characterization
- 5.3.1. Cell Line Characterization Methods
- 5.3.1.1. Identity / Stability Testing
- 5.3.1.1.1. Karyotype Analysis
- 5.3.1.1.2. Cytochrome C Oxidase I Barcoding Assay
- 5.3.1.1.3. Cell Morphology Analysis
- 5.3.1.1.4. DNA Analysis
- 5.3.1.2. Sterility / Biosafety Testing
- 5.3.1.2.1. Mycoplasma Contamination Testing
- 5.3.1.2.2. Viral Contamination Testing
- 5.3.1.1. Identity / Stability Testing
- 5.3.1. Cell Line Characterization Methods
- 5.4. Applications of Cell Lines
- 5.5. Key Concerns Associated with Cell Lines
- 5.6. Need for Outsourcing Cell Line Related Operations
- 5.7. Concluding Remarks
6. CELL LINE DEVELOPMENT SERVICE PROVIDERS: MARKET LANDSCAPE
- 6.1. Chapter Overview
- 6.2. Cell Line Development Service Providers: Overall Market Landscape
- 6.2.1. Analysis by Year of Establishment
- 6.2.2. Analysis by Company Size
- 6.2.3. Analysis by Location of Headquarters
- 6.2.4. Analysis by Company Size and Location of Headquarters
- 6.2.5. Analysis by Source of Cell Line / Expression System Offered
- 6.2.6. Analysis by Gene Delivery Method Used
- 6.2.7. Analysis by Type of Transfection
- 6.2.8. Analysis by Availability of Serum-Free / Animal Component Free Culturing Capability
- 6.2.9. Analysis by Type of Cells Offered
- 6.2.10. Analysis by Application of Cell Lines
- 6.2.11. Analysis by Additional Cell Line Related Services Offered
- 6.2.12. Analysis by Type of Cell Banking
- 6.2.13. Analysis by Availability of Integrated Cell Line Characterization Service
7. CELL LINE DEVELOPMENT SERVICE PROVIDERS: COMPANY COMPETITIVENESS ANALYSIS
- 7.1. Chapter Overview
- 7.2. Assumptions / Key Parameters
- 7.3. Methodology
- 7.4. Cell Line Development Service Providers: Company Competitiveness Analysis
- 7.4.1. Cell Line Development Service Providers in North America
- 7.4.2. Cell Line Development Service Providers in Europe
- 7.4.3. Cell Line Development Service Providers in Asia-Pacific
8. COMPANY PROFILES: CELL LINE DEVELOPMENT SERVICE PROVIDERS
- 8.1. Chapter Overview
- 8.2. Detailed Company Profiles of Leading Service Providers
- 8.2.1. ATUM
- 8.2.1.1. Company Overview
- 8.2.1.2. Cell Line Development Service Portfolio
- 8.2.1.3. Recent Developments and Future Outlook
- 8.2.2. Curia
- 8.2.2.1. Company Overview
- 8.2.2.2. Cell Line Development Service Portfolio
- 8.2.2.3. Recent Developments and Future Outlook
- 8.2.3. Fujifilm Diosynth Biotechnologies
- 8.2.3.1. Company Overview
- 8.2.3.2. Service Portfolio for Cell Line Development
- 8.2.3.3. Recent Developments and Future Outlook
- 8.2.4. Lonza
- 8.2.4.1. Company Overview
- 8.2.4.2. Cell Line Development Service Portfolio
- 8.2.4.3. Recent Developments and Future Outlook
- 8.2.5. Syngene International
- 8.2.5.1. Company Overview
- 8.2.5.2. Cell Line Development Service Portfolio
- 8.2.5.3. Recent Developments and Future Outlook
- 8.2.6. WuXi Biologics
- 8.2.6.1. Company Overview
- 8.2.6.2. Cell Line Development Service Portfolio
- 8.2.6.3. Recent Developments and Future Outlook
- 8.2.1. ATUM
- 8.3. Short Profiles of Other Prominent Service Providers
- 8.3.1. Biovian
- 8.3.1.1. Company Overview
- 8.3.1.2. Cell Line Development Service Portfolio
- 8.3.2. Celonic
- 8.3.2.1. Company Overview
- 8.3.2.2. Cell Line Development Service Portfolio
- 8.3.3. ChemPartner
- 8.3.3.1. Company Overview
- 8.3.3.2. Cell Line Development Service Portfolio
- 8.3.4. Creative Biogene
- 8.3.4.1. Company Overview
- 8.3.4.2. Cell Line Development Service Portfolio
- 8.3.5. KBI Biopharma
- 8.3.5.1. Company Overview
- 8.3.5.2. Cell Line Development Service Portfolio
- 8.3.6. Kemp Proteins
- 8.3.6.1. Company Overview
- 8.3.6.2. Cell Line Development Service Portfolio
- 8.3.7. KMD Bioscience
- 8.3.7.1. Company Overview
- 8.3.7.2. Cell Line Development Service Portfolio
- 8.3.8. Mycenax Biotech
- 8.3.8.1. Company Overview
- 8.3.8.2. Cell Line Development Service Portfolio
- 8.3.9. ProBioGen
- 8.3.9.1. Company Overview
- 8.3.9.2. Cell Line Development Service Portfolio
- 8.3.10. Thermo Fisher Scientific
- 8.3.10.1. Company Overview
- 8.3.10.2. Cell Line Development Service Portfolio
- 8.3.1. Biovian
9. CELL LINE CHARACTERIZATION SERVICE PROVIDERS: MARKET LANDSCAPE
- 9.1. Chapter Overview
- 9.2. Cell Line Characterization Service Providers: Overall Market Landscape
- 9.2.1. Cell Line Characterization Service Providers Service Providers (Industry Players)
- 9.2.1.1. Analysis by Year of Establishment
- 9.2.1.2. Analysis by Company Size
- 9.2.1.3. Analysis by Location of Headquarters
- 9.2.1.4. Analysis by Location of Headquarters and Company Size
- 9.2.1.5. Analysis by Cell line Characterized
- 9.2.1.6. Analysis by Type of Cell Line Characterization Service Offered
- 9.2.1.6.1. Analysis by Type of Cell Line Identity / Stability Testing Service Offered
- 9.2.1.6.2. Analysis by Type of Cell Line Sterility / Biosafety Testing Service Offered
- 9.2.2. Cell Line Characterization Service Providers (Non-Industry Players)
- 9.2.2.1. Analysis by Location of Organization
- 9.2.2.2. Analysis by Types of Cell Line Characterized
- 9.2.2.3. Analysis by Type of Cell Line Characterization Service Offered
- 9.2.2.4. Analysis by Genotyping Kit Used
- 9.2.2.5. Analysis by Number of Loci Amplified
- 9.2.1. Cell Line Characterization Service Providers Service Providers (Industry Players)
10. CELL LINE CHARACTERIZATION SERVICE PROVIDERS: COMPANY COMPETITIVENESS ANALYSIS
- 10.1. Chapter Overview
- 10.2. Assumptions / Key Parameters
- 10.3. Methodology
- 10.4. Cell Line Characterization Service Providers: Company Competitiveness Analysis
- 10.4.1. Cell Line Characterization Service Providers in North America
- 10.4.2. Cell Line Characterization Service Providers in Europe
- 10.4.3. Cell Line Characterization Service Providers in Asia-Pacific
11. COMPANY PROFILES: CELL LINE CHARACTERIZATION SERVICE PROVIDERS
- 11.1. Chapter Overview
- 11.2. Detailed Company Profiles of Leading Service Provider
- 11.2.1 Charles River Laboratories
- 11.2.1.1. Company Overview
- 11.2.1.2. Cell Line Characterization Service Portfolio
- 11.2.1.3. Recent Developments and Future Outlook
- 11.2.2. Eurofins BioPharma Product Testing
- 11.2.2.1. Company Overview
- 11.2.2.2. Cell Line Characterization Service Portfolio
- 11.2.2.3. Recent Developments and Future Outlook
- 11.2.3. Livogen Pharmed
- 11.2.3.1. Company Overview
- 11.2.3.2. Cell Line Characterization Service Portfolio
- 11.2.3.3. Recent Developments and Future Outlook
- 11.2.4. Molecular Diagnostic Services
- 11.2.4.1. Company Overview
- 11.2.4.2. Cell Line Characterization Service Portfolio
- 11.2.4.3. Recent Developments and Future Outlook
- 11.2.5. Sartorius
- 11.2.5.1. Company Overview
- 11.2.5.2. Cell Line Characterization Service Portfolio
- 11.2.5.3. Recent Developments and Future Outlook
- 11.2.6. TFBS Bioscience
- 11.2.6.1. Company Overview
- 11.2.6.2. Cell Line Characterization Service Portfolio
- 11.2.6.3. Recent Developments and Future Outlook
- 11.2.1 Charles River Laboratories
- 11.3. Short Profiles of Other Prominent Service Providers
- 11.3.1. ATZ Labs (Subsidiary of Life Technologies)
- 11.3.1.1. Company Overview
- 11.3.1.2. Cell Line Characterization Service Portfolio
- 11.3.2. Avance Biosciences
- 11.3.2.1. Company Overview
- 11.3.2.2. Cell Line Characterization Service Portfolio
- 11.3.3. BioReliance
- 11.3.3.1. Company Overview
- 11.3.3.2. Cell Line Characterization Service Portfolio
- 11.3.4. Clean Cells
- 11.3.4.1. Company Overview
- 11.3.4.2. Cell Line Characterization Service Portfolio
- 11.3.5. hylabs
- 11.3.5.1. Company Overview
- 11.3.5.2. Cell Line Characterization Service Portfolio
- 11.3.6. Samsung Biologics
- 11.3.6.1. Company Overview
- 11.3.6.2. Cell Line Characterization Service Portfolio
- 11.3.7. SGS Life Sciences
- 11.3.7.1. Company Overview
- 11.3.7.2. Cell Line Characterization Service Portfolio
- 11.3.8. Texcell
- 11.3.8.1. Company Overview
- 11.3.8.2. Cell Line Characterization Service Portfolio
- 11.3.9. WuXi Advanced Therapies
- 11.3.9.1. Company Overview
- 11.3.9.2. Cell Line Characterization Service Portfolio
- 11.3.1. ATZ Labs (Subsidiary of Life Technologies)
12. PARTNERSHIPS AND COLLABORATIONS
- 12.1. Chapter Overview
- 12.2. Partnership Models
- 12.3. Cell Line Development and Characterization: Partnerships and Collaborations
- 12.3.1. Analysis by Year of Partnership
- 12.3.2. Analysis by Type of Partnership
- 12.3.3. Analysis by Year and Type of Partnership
- 12.3.4. Analysis by Type of Partner
- 12.3.5. Most Active Players: Analysis by Number of Partnerships
- 12.3.6. Analysis by Geography
- 12.3.6.1. Intracontinental and Intercontinental Deals
- 12.3.6.2. International and Local Deals
13. RECENT EXPANSIONS
- 13.1. Chapter Overview
- 13.2. Cell Line Development and Characterization: List of Recent Expansions
- 13.2.1. Analysis by Year of Expansion
- 13.2.2. Analysis by Type of Expansion
- 13.2.3. Analysis by Year and Type of Expansion
- 13.2.4. Analysis by Location of Headquarters and Company Size
- 13.2.5. Analysis by Location of Expanded Facility
- 13.2.6. Analysis by Type of Expansion and Location of Expanded Facility
- 13.2.7. Analysis by Area of Expanded Facility (sq ft)
- 13.2.8. Analysis by Purpose of Expansion
- 13.2.9. Analysis by Geography
- 13.2.10. Most Active Players: Analysis by Number of Recent Expansions
14. CELL LINE REPOSITORIES
- 14.1. Chapter Overview
- 14.2. American Type Culture Collection (ATCC)
- 14.2.1. Overview
- 14.2.2. Service Portfolio
- 14.3. Coriell Institute for Medical Research
- 14.3.1. Overview
- 14.3.2. Service Portfolio
- 14.4. European Collection of Authenticated Cell Cultures (ECACC)
- 14.4.1. Overview
- 14.4.2. Service Portfolio
- 14.5. Leibniz Institute DSMZ - German Collection of Microorganisms and Cell Cultures
- 14.5.1. Overview
- 14.5.2. Service Portfolio
- 14.6. National Centre for Cell Science (NCCS)
- 14.6.1. Overview
- 14.6.2. Service Portfolio
15. REGULATORY RECOMMENDATIONS AND GUIDELINES
- 15.1. Chapter Overview
- 15.2. Cell Line Authentication: Prominent Regulatory Authorities
- 15.2.1. Role of American Type Culture Collection (ATCC)
- 15.2.2. Role of International Cell Line Authentication Committee (ICLAC)
- 15.2.3. Role of Global Biological Standards Institute (GBSI)
- 15.3. Guidelines Issued by International Regulatory Agencies
- 15.3.1. World Health Organization
- 15.3.2. The International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH)
- 15.4. Regulatory Guidelines for Cell Line Characterization in the US
- 15.4.1. Food and Drug Administration
- 15.4.1.1. FDA Points to Consider
- 15.4.1.2. US Pharmacopeial Convention
- 15.4.1.3. US Code for Federal Regulations
- 15.4.1. Food and Drug Administration
- 15.5. Regulatory Guidelines for Cell Line Characterization in Europe
- 15.5.1. European Medicines Agency
- 15.6. Regulatory Guidelines for Cell Line Characterization In Japan
- 15.6.1. Ministry of Health, Labor and Welfare
- 15.7. Concluding Remarks
16. MARKET IMPACT ANALYSIS: DRIVERS, RESTRAINTS, OPPORTUNITIES AND CHALLENGES
- 16.1. Chapter Overview
- 16.2. Market Drivers
- 16.3. Market Restraints
- 16.4. Market Opportunities
- 16.5. Market Challenges
- 16.6. Conclusion
17. GLOBAL CELL LINE DEVELOPMENT SERVICES MARKET
- 17.1. Chapter Overview
- 17.2. Assumptions and Methodology
- 17.3. Global Cell Line Development Services Market, Historical Trends (Since 2019) and Forecasted Estimates (Till 2035)
- 17.3.1. Scenario Analysis
- 17.3.1.1. Conservative Scenario
- 17.3.1.2. Optimistic Scenario
- 17.3.1. Scenario Analysis
- 17.4. Key Market Segmentations
18. CELL LINE DEVELOPMENT SERVICES MARKET, BY SOURCE OF CELL LINES
- 18.1. Chapter Overview
- 18.2. Key Assumptions and Methodology
- 18.3. Cell Line Development Services Market: Distribution by Source of Cell Lines
- 18.3.1. Cell Line Development Services Market for Mammalian Cell Lines: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035)
- 18.3.2. Cell Line Development Services Market for Microbial Cell Lines: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035)
- 18.3.3. Cell Line Development Services Market for Insect Cell Lines: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035)
- 18.3.4. Cell Line Development Services Market for Other Cell Lines: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035)
- 18.4. Data Triangulation and Validation
19. CELL LINE DEVELOPMENT SERVICES MARKET, BY APPLICATION OF CELL LINES
- 19.1. Chapter Overview
- 19.2. Key Assumptions and Methodology
- 19.3. Cell Line Development Services Market: Distribution by Application of Cell Lines
- 19.3.1. Cell Line Development Services Market for Biomanufacturing: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035)
- 19.3.2. Cell Line Development Services Market for Research and Development Operations: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035)
- 19.4. Data Triangulation and Validation
20. CELL LINE DEVELOPMENT SERVICES MARKET, BY COMPANY SIZE
- 20.1. Chapter Overview
- 20.2. Key Assumptions and Methodology
- 20.3. Cell Line Development Services Market: Distribution by Company Size
- 20.3.1. Cell Line Development Services Market for Large Companies: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035)
- 20.3.2. Cell Line Development Services Market for Mid-sized Companies: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035)
- 20.3.3. Cell Line Development Services Market for Small Companies: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035)
- 20.4. Data Triangulation and Validation
21. CELL LINE DEVELOPMENT SERVICES MARKET, BY KEY GEOGRAPHICAL REGIONS
- 21.1. Chapter Overview
- 21.2. Key Assumptions and Methodology
- 21.3. Cell Line Development Services Market: Distribution by Company Size
- 21.3.1. Cell Line Development Services Market for North America: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035)
- 21.3.2. Cell Line Development Services Market for Europe: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035)
- 21.3.3. Cell Line Development Services Market for Asia-Pacific and Rest of the World: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035)
- 21.4. Data Triangulation and Validation
22. GLOBAL CELL LINE CHARACTERIZATION SERVICES MARKET
- 22.1. Chapter Overview
- 22.2. Assumptions and Methodology
- 22.3. Global Cell Line Characterization Services Market, Historical Trends (Since 2019) and Forecasted Estimates (Till 2035)
- 22.3.1. Scenario Analysis
- 22.3.1.1. Conservative Scenario
- 22.3.1.2. Optimistic Scenario
- 22.3.1. Scenario Analysis
- 22.4. Key Market Segmentations
23. CELL LINE CHARACTERIZATION SERVICES MARKET, BY SOURCE OF CELL LINES
- 23.1. Chapter Overview
- 23.2. Key Assumptions and Methodology
- 23.3. Cell Line Characterization Services Market: Distribution by Source of Cell Lines
- 23.3.1. Cell Line Characterization Services Market for Mammalian Cell Lines: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035)
- 23.3.2. Cell Line Characterization Services Market for Microbial Cell Lines: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035)
- 23.3.3. Cell Line Characterization Services Market for Insect Cell Lines: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035)
- 23.3.4. Cell Line Characterization Services Market for Other Cell Lines: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035)
- 23.4. Data Triangulation and Validation
24. CELL LINE CHARACTERIZATION SERVICES MARKET, BY APPLICATION OF CELL LINES
- 24.1. Chapter Overview
- 24.2. Key Assumptions and Methodology
- 24.3. Cell Line Characterization Services Market: Distribution by Application of Cell Lines
- 24.3.1. Cell Line Characterization Services Market for Biomanufacturing: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035)
- 24.3.2. Cell Line Characterization Services Market for Research and Development Operations: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035)
- 24.4. Data Triangulation and Validation
25. CELL LINE CHARACTERIZATION SERVICES MARKET, BY COMPANY SIZE
- 25.1. Chapter Overview
- 25.2. Key Assumptions and Methodology
- 25.3. Cell Line Characterization Services Market: Distribution by Company Size
- 25.3.1. Cell Line Characterization Services Market for Large Companies: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035)
- 25.3.2. Cell Line Characterization Services Market for Mid-sized Companies: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035)
- 25.3.3. Cell Line Characterization Services Market for Small Companies: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035)
- 25.4. Data Triangulation and Validation
26. CELL LINE CHARACTERIZATION SERVICES MARKET, BY KEY GEOGRAPHICAL REGIONS
- 26.1. Chapter Overview
- 26.2. Key Assumptions and Methodology
- 26.3. Cell Line Characterization Services Market: Distribution by Key Geographical Regions
- 26.3.1. Cell Line Characterization Services Market for North America: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035)
- 26.3.2. Cell Line Characterization Services Market for Europe: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035)
- 26.3.3. Cell Line Characterization Services Market for Asia-Pacific and Rest of the World: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035)
- 26.4. Data Triangulation and Validation
27. CONCLUDING REMARKS
28. EXECUTIVE INSIGHTS
- 28.1. Chapter Overview
- 28.2. Company A
- 28.2.1. Company Snapshot
- 28.2.2. Interview Transcript, Chief Executive Officer
- 28.3. Company B
- 28.3.1. Company Snapshot
- 28.3.2. Interview Transcript, Principal Scientist and Head of R&D
- 28.4. Company C
- 28.4.1. Company Snapshot
- 28.4.2. Interview Transcript, Director of Business Development and Marketing
- 28.5. Company D
- 28.5.1. Company Snapshot
- 28.5.2. Interview Transcript, Founder and President
- 28.6. Company E
- 28.6.1. Company Snapshot
- 28.6.2. Interview Transcript, President
- 28.7. Company F
- 28.7.1. Company Snapshot
- 28.7.2. Interview Transcript, Chief Scientific Officer
- 28.8. Company G
- 28.8.1. Company Snapshot
- 28.8.2. Interview Transcript, Former Vice President BioProcessing
- 28.9. Company H
- 28.9.1. Company Snapshot
- 28.9.2. Interview Transcript, Founder and Managing Director
- 28.10. Company I
- 28.10.1. Company Snapshot
- 28.10.2. Interview Transcript, Client Relations Manager
- 28.11. Company J
- 28.11.1. Company Snapshot
- 28.11.2. Interview Transcript, Former Business Development Manager
29. APPENDIX 1: TABULATED DATA
30. APPENDIX 2: LIST OF COMPANIES AND ORGANIZATIONS
31. APPENDIX 3: DETAILS OF PARTNERSHIPS AND COLLABORATIONS
List of Tables
- Table 5.1 Comparison between Finite and Continuous Cell Lines
- Table 5.2 Popular Mammalian Cell Lines used in Biopharmaceutical Production
- Table 5.3 Human Cell Lines and Therapeutic Protein Products
- Table 5.4 Cell Lines and Viral Vaccine Products
- Table 6.1 Cell Line Development: List of Service Providers
- Table 6.2 Cell Line Development Service Providers: Information on Source of Cell Line / Expression System Offered, Gene Delivery Method Used and Type of Transfection
- Table 6.3 Cell Line Development Service Providers: Information on Availability Serum free / Animal Component Free Culturing Capability, Types of Cells Offered and Applications of Cell Lines
- Table 6.4 Cell Line Development Service Providers: Information on Additional Cell Line Related Services Offered, Type of Cell Banking and Integrated Cell Line Characterization Service
- Table 6.5 Cell Line Development Service Providers: Information on Technology/Cell Line / Vectors / Expression System Used (if available), Availability of Monoclonality Procedure, Availability of Biosimilar Cell Line Development Service and Availability of Genetically Edited Cell Line Development Service
- Table 8.1 Cell Line Development Service Providers: List of Companies Profiled
- Table 8.2 ATUM: Company Snapshot
- Table 8.3 ATUM: Cell Line Development Service Portfolio
- Table 8.4 ATUM: Recent Developments and Future Outlook
- Table 8.5 Curia: Company Snapshot
- Table 8.6 Curia: Cell Line Development Service Portfolio
- Table 8.7 Curia: Recent Developments and Future Outlook
- Table 8.8 FUJIFILM Diosynth Biotechnologies: Company Snapshot
- Table 8.9 FUJIFILM Diosynth Biotechnologies: Cell Line Development Service Portfolio
- Table 8.10 FUJIFILM Diosynth Biotechnologies: Recent Developments and Future Outlook
- Table 8.11 Lonza: Company Snapshot
- Table 8.12 Lonza: Cell Line Development Service Portfolio
- Table 8.13 Lonza: Recent Developments and Future Outlook
- Table 8.14 Syngene International: Company Snapshot
- Table 8.15 Syngene International: Cell Line Development Service Portfolio
- Table 8.16 Syngene International: Recent Developments and Future Outlook
- Table 8.17 WuXi Biologics: Company Snapshot
- Table 8.18 WuXi Biologics: Cell Line Development Service Portfolio
- Table 8.19 WuXi Biologics: Recent Developments and Future Outlook
- Table 8.20 Biovian: Company Snapshot
- Table 8.21 Biovian: Cell Line Development Service Portfolio
- Table 8.22 Celonic: Company Snapshot
- Table 8.23 Celonic: Cell Line Development Service Portfolio
- Table 8.24 ChemPartner: Company Snapshot
- Table 8.25 ChemPartner: Cell Line Development Service Portfolio
- Table 8.26 Creative Biogene: Company Snapshot
- Table 8.27 Creative Biogene: Cell Line Development Service Portfolio
- Table 8.28 KBI Biopharma: Company Snapshot
- Table 8.29 KBI Biopharma: Cell Line Development Service Portfolio
- Table 8.30 Kemp Proteins: Company Snapshot
- Table 8.31 Kemp Proteins: Cell Line Development Service Portfolio
- Table 8.32 KMD Bioscience: Company Snapshot
- Table 8.33 KMD Bioscience: Cell Line Development Service Portfolio
- Table 8.34 Mycenax Biotech: Company Snapshot
- Table 8.35 Mycenax Biotech: Cell Line Development Service Portfolio
- Table 8.36 ProBioGen: Company Snapshot
- Table 8.37 ProBioGen: Cell Line Development Service Portfolio
- Table 8.38 Thermo Fisher Scientific: Company Snapshot
- Table 8.39 Thermo Fisher Scientific: Cell Line Development Service Portfolio
- Table 9.1 Cell Line Characterization: List of Service Providers (Industry Players)
- Table 9.2 Cell line Development Service Providers: Information on Cell Line Characterized and Type of Cell Line Characterization Services Offered
- Table 9.3 Cell Line Characterization Service Providers: Information on Type of Cell Line Identity/ Stability Testing Services Offered and Type of Cell Line Sterility / Biosafety Testing Services Offered
- Table 9.4 Cell line Characterization Service Providers: Information on Regulatory Compliance Regulatory Accreditations /Certifications Overall Turnaround Time, and Availability of Additional Cell Line Related Services
- Table 9.5 Cell Line Characterization: List of Service Providers (Non-Industry Players)
- Table 9.6 Cell Line Characterization Service Providers: Information on Type of Cell Line Characterization Service Offered and Type of Genotyping Services Offered
- Table 9.7 Cell Line Characterization Service Providers: Information on Genotyping Kit Used, Number of Loci Amplified, Service Charges and Overall Turnaround Time
- Table 11.1 Cell Line Characterization Service Providers: List of Companies Profiled
- Table 11.2 Charles River Laboratories: Company Snapshot
- Table 11.3 Charles River laboratories: Cell Line Characterization Service Portfolio
- Table 11.4 Charles River Laboratories: Recent Developments and Future Outlook
- Table 11.5 Eurofins BioPharma Product Testing: Company Snapshot
- Table 11.6 Eurofins BioPharma Product Testing: Cell Line Characterization Service Portfolio
- Table 11.7 Eurofins BioPharma Product Testing: Recent Developments and Future Outlook
- Table 11.8 Livogen Pharmed: Company Snapshot
- Table 11.9 Livogen Pharmed: Cell Line Characterization Service Portfolio
- Table 11.10 Molecular Diagnostic Services: Company Snapshot
- Table 11.11 Molecular Diagnostic Services: Cell Line Characterization Service Portfolio
- Table 11.12 Sartorius: Company Snapshot
- Table 11.13 Sartorius: Cell Line Characterization Service Portfolio
- Table 11.14 Sartorius: Recent Developments and Future Outlook
- Table 11.15 TFBS Bioscience: Company Snapshot
- Table 11.16 TFBS Bioscience: Cell Line Characterization Service Portfolio
- Table 11.17 TFBS Bioscience: Recent Developments and Future Outlook
- Table 11.18 ATZ Labs: Company Snapshot
- Table 11.19 ATZ Labs: Cell Line Characterization Service Portfolio
- Table 11.20 Avance Biosciences: Company Snapshot
- Table 11.21 Avance Biosciences: Cell Line Characterization Service Portfolio
- Table 11.22 BioReliance: Company Snapshot
- Table 11.23 BioReliance: Cell Line Characterization Service Portfolio
- Table 11.24 Clean Cells: Company Snapshot
- Table 11.25 Clean Cells: Cell Line Characterization Service Portfolio
- Table 11.26 Hylabs: Company Snapshot
- Table 11.27 Hylabs: Cell Line Characterization Service Portfolio
- Table 11.28 Samsung Biologics: Company Snapshot
- Table 11.29 Samsung Biologics: Cell Line Characterization Service Portfolio
- Table 11.30 SGS Life Sciences: Company Snapshot
- Table 11.31 SGS Life Sciences: Cell Line Characterization Service Portfolio
- Table 11.32 Texcell: Company Snapshot
- Table 11.33 Texcell: Cell Line Characterization Service Portfolio
- Table 11.34 WuXi Advanced Therapies: Company Snapshot
- Table 11.35 WuXi Advanced Therapies: Cell Line Characterization Service Portfolio
- Table 12.1 Cell Line Development and Characterization: List of Partnerships and Collaborations, Since 2019
- Table 13.1 Cell Line Development and Characterization Services: List of Recent Expansions, Since 2019
- Table 15.1 List of Journals with Mandatory Requirement of Cell Line Authentication for Research Submissions
- Table 15.2 International Cell Line Authentication Committee (ICLAC): Partner Organizations
- Table 15.3 WHO Recommendations: Cell Bank Characterization
- Table 15.4 Regulatory Documents for Cell Line Characterization in the US
- Table 15.5 US Pharmacopoeia (USP) 1050: Virus Testing in Cell Banks
- Table 15.6 European Pharmacopoeias: Testing in Cell Banks
- Table 28.1 Biofortuna: Company Snapshot
- Table 28.2 Abgenex: Company Snapshot
- Table 28.3 GTP Bioways: Company Snapshot
- Table 28.4 QuaCell Biotechnology: Company Snapshot
- Table 28.5 Laragen: Company Snapshot
- Table 28.6 Polpharma Biologics: Company Snapshot
- Table 28.7 LakePharam (Acquired by Curia): Company Snapshot
- Table 28.8 ARTES Biotechnology: Company Snapshot
- Table 28.9 ImmunoPrecise Antibodies (Formerly Modiquest): Company Snapshot
- Table 28.10 Polpharma Biologics: Company Snapshot
- Table 29.1 Cell Line Development Service Providers: Distribution by Year of Establishment
- Table 29.2 Cell Line Development Service Providers: Distribution by Company Size
- Table 29.3 Cell Line Development Service Providers: Distribution by Location of Headquarters
- Table 29.4 Cell Line Development Service Providers: Distribution by Company Size and Location of Headquarters
- Table 29.5 Cell Line Development Service Providers: Distribution by Source of Cell Line / Expression System Offered
- Table 29.6 Cell Line Development Service Providers: Distribution by Gene Delivery Method Used
- Table 29.7 Cell Line Development Service Providers: Distribution by Type of Transfection
- Table 29.8 Cell Line Development Service Providers: Distribution by Availability of Serum-Free /Animal Component Free Culturing Capability
- Table 29.9 Cell Line Development Service Providers: Distribution by Type of Cells Offered
- Table 29.10 Cell Line Development Service Providers: Distribution by Application of Cell Lines
- Table 29.11 Cell Line Development Service Providers: Distribution by Additional Cell Line Related Services Offered
- Table 29.12 Cell Line Development Service Providers: Distribution by Type of Cell Banking
- Table 29.13 Cell Line Development Service Providers: Distribution by Availability of Integrated Cell Line Characterization Service
- Table 29.14 Cell Line Characterization Service Providers: Overall Market Landscape
- Table 29.15 Cell Line Characterization Service Providers (Industry): Distribution by Year of Establishment
- Table 29.16 Cell Line Characterization Service Providers (Industry): Distribution by Company Size
- Table 29.17 Cell Line Characterization Service Providers (Industry): Distribution by Location of Headquarters
- Table 29.18 Cell Line Characterization Service Providers (Industry): Distribution by Company Size and Location of Headquarters
- Table 29.19 Cell Line Characterization Service Providers (Industry): Distribution by Cell Line Characterized
- Table 29.20 Cell Line Characterization Service Providers (Industry): Distribution by Type of Cell Line Characterization Services Offered
- Table 29.21 Cell Line Characterization Service Providers (Industry): Distribution by Type of Cell Line Identity / Stability Testing Service Offered
- Table 29.22 Cell Line Characterization Service Providers (Industry): Distribution by Type of Cell Line Sterility / Biosafety Testing Service Offered
- Table 29.23 Cell Line Characterization Service Providers (Non-Industry): Distribution by Location of Organization
- Table 29.24 Cell Line Characterization Service Providers (Non-Industry): Distribution by Type of Cell Line Characterized
- Table 29.25 Cell Line Characterization Service Providers (Non-Industry): Distribution by Type of Cell Line Characterization Service Offered
- Table 29.26 Cell Line Characterization Service Providers (Non-Industry): Distribution by Genotyping Kit Used
- Table 29.27 Cell Line Characterization Service Providers (Non-Industry): Distribution by Number of Loci Amplified
- Table 29.28 Partnerships and Collaborations: Cumulative Year-wise Trend, Since 2015
- Table 29.29 Partnerships and Collaborations: Distribution by Type of Partnership
- Table 29.30 Partnerships and Collaborations: Distribution by Year and Type of Partnership
- Table 29.31 Partnerships and Collaborations: Distribution by Type of Partner
- Table 29.32 Most Active Players: Distribution by Number of Partnerships
- Table 29.33 Partnerships and Collaborations: Distribution by Intracontinental and Intercontinental Deals
- Table 29.34 Partnerships and Collaborations: Local and International Deals
- Table 29.35 Recent Expansions: Cumulative Year-wise Trend, Since 2023
- Table 29.36 Recent Expansions: Distribution by Type of Expansion
- Table 29.37 Recent Expansions: Distribution by Year and Type of Expansion
- Table 29.38 Recent Expansions: Distribution by Location of Headquarters and Company Size
- Table 29.39 Recent Expansions: Distribution by Location of Expanded Facility
- Table 29.40 Recent Expansions: Distribution by Type of Expansion and Location of Expanded Facility
- Table 29.41 Recent Expansions: Distribution by Area of Expanded Facility (sq. ft.)
- Table 29.42 Recent Expansions: Distribution by Purpose of Expansion
- Table 29.43 Recent Expansions: Analysis by Geography
- Table 29.44 Most Active Players: Distribution by Number of Recent Expansions
- Table 29.45 Global Cell Line Development Services Market, Historical Trends (Since 2019) (USD Million)
- Table 29.46 Global Cell Line Development Services Market, Forecasted Estimates (Till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 29.47 Cell Line Development Services Market: Distribution by Source of Cell Line (USD Million)
- Table 29.48 Cell Line Development Services Market for Mammalian Cell Lines: Historical Trends (Since 2019) (USD Million)
- Table 29.49 Cell Line Development Services Market for Mammalian Cell Lines: Forecasted Estimates (Till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 29.50 Cell Line Development Services Market for Microbial Cell Lines: Historical Trends (Since 2019) (USD Million)
- Table 29.51 Cell Line Development Services Market for Microbial Cell Lines: Forecasted Estimates (Till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 29.52 Cell Line Development Services Market for Insect Cell Lines: Historical Trends (Since 2019) (USD Million)
- Table 29.53 Cell Line Development Services Market for Insect Cell Lines: Forecasted Estimates (Till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 29.54 Cell Line Development Services Market for Other Cell Lines: Historical Trends (Since 2019) (USD Million)
- Table 29.55 Cell Line Development Services Market for Other Cell Lines: Forecasted Estimates (Till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 29.56 Cell Line Development Services Market: Distribution by Application of Cell Line (USD Million)
- Table 29.57 Cell Line Development Services Market for Biomanufacturing: Historical Trends (Since 2019) (USD Million)
- Table 29.58 Cell Line Development Services Market for Biomanufacturing: Forecasted Estimates (Till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 29.59 Cell Line Development Services Market for Research and Development Operations: Historical Trends (Since 2019) (USD Million)
- Table 29.60 Cell Line Development Services Market for Research and Development Operations: Forecasted Estimates (Till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 29.61 Cell Line Development Services Market: Distribution by Company Size (USD Million)
- Table 29.62 Cell Line Development Services Market for Large Companies: Historical Trends (Since 2019) (USD Million)
- Table 29.63 Cell Line Development Services Market for Large Companies: Forecasted Estimates (Till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 29.64 Cell Line Development Services Market for Mid-Size Companies: Historical Trends (Since 2019) (USD Million)
- Table 29.65 Cell Line Development Services Market for Mid-sized Companies: Forecasted Estimates (Till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 29.66 Cell Line Development Services Market for Small Companies: Historical Trends (Since 2019) (USD Million)
- Table 29.67 Cell Line Development Services Market for Small Companies: Forecasted Estimates (Till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 29.68 Cell Line Development Services Market: Distribution by Key Geographical Regions (USD Million)
- Table 29.69 Cell Line Development Services Market for North America: Historical Trends (Since 2019) (USD Million)
- Table 29.70 Cell Line Development Services Market for North America: Forecasted Estimates (Till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 29.71 Cell Line Development Services Market for Europe: Historical Trends (Since 2019) (USD Million)
- Table 29.72 Cell Line Development Services Market for Europe: Forecasted Estimates (Till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 29.73 Cell Line Development Services Market for Asia-Pacific and Rest of the World: Historical Trends (Since 2019) (USD Million)
- Table 29.74 Cell Line Development Services Market for Asia-Pacific and Rest of the World: Forecasted Estimates (Till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 29.75 Global Cell Line Characterization Services Market, Historical Trends (Since 2019) (USD Million)
- Table 29.76 Global Cell Line Characterization Services Market, Forecasted Estimates (Till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 29.77 Cell Line Characterization Services Market: Distribution by Source of Cell Line (USD Million)
- Table 29.78 Cell Line Characterization Services Market for Mammalian Cell Lines: Historical Trends (Since 2019) (USD Million)
- Table 29.79 Cell Line Characterization Services Market for Mammalian Cell Lines: Forecasted Estimates (Till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 29.80 Cell Line Characterization Services Market for Microbial Cell Lines: Historical Trends (Since 2019) (USD Million)
- Table 29.81 Cell Line Characterization Services Market for Microbial Cell Lines: Forecasted Estimates (Till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 29.82 Cell Line Characterization Services Market for Insect Cell Lines: Historical Trends (Since 2019) (USD Million)
- Table 29.83 Cell Line Characterization Services Market for Insect Cell Lines: Forecasted Estimates (Till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 29.84 Cell Line Characterization Services Market for Other Cell Lines: Historical Trends (Since 2019) (USD Million)
- Table 29.85 Cell Line Characterization Services Market for Other Cell Lines: Forecasted Estimates (Till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 29.86 Cell Line Characterization Services Market: Distribution by Application of Cell Line (USD Million)
- Table 29.87 Cell Line Characterization Services Market for Biomanufacturing: Historical Trends (Since 2019) (USD Million)
- Table 29.88 Cell Line Characterization Services Market for Biomanufacturing: Forecasted Estimates (Till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 29.89 Cell Line Characterization Services Market for Research and Development Operations: Historical Trends (Since 2019) (USD Million)
- Table 29.90 Cell Line Characterization Services Market for Research and Development Operations: Forecasted Estimates (Till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 29.91 Cell Line Characterization Services Market: Distribution by Company Size (USD Million)
- Table 29.92 Cell Line Characterization Services Market for Large Companies: Historical Trends (Since 2019) (USD Million)
- Table 29.93 Cell Line Characterization Services Market for Large Companies: Forecasted Estimates (Till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 29.94 Cell Line Characterization Services Market for Mid-Size Companies: Historical Trends (Since 2019) (USD Million)
- Table 29.95 Cell Line Characterization Services Market for Mid-sized Companies: Forecasted Estimates (Till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 29.96 Cell Line Characterization Services Market for Small Companies: Historical Trends (Since 2019) (USD Million)
- Table 29.97 Cell Line Characterization Services Market for Small Companies: Forecasted Estimates (Till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 29.98 Cell Line Characterization Services Market: Distribution by Key Geographical Regions (USD Million)
- Table 29.99 Cell Line Characterization Services Market for North America: Historical Trends (Since 2019) (USD Million)
- Table 29.100 Cell Line Characterization Services Market for North America: Forecasted Estimates (Till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 29.101 Cell Line Characterization Services Market for Europe: Historical Trends (Since 2019) (USD Million)
- Table 29.102 Cell Line Characterization Services Market for Europe: Forecasted Estimates (Till 2035), Conservative, Base and Optimistic Scenario (USD Million)
- Table 29.103 Cell Line Characterization Services Market for Asia-Pacific and Rest of the World: Historical Trends (Since 2019) (USD Million)
- Table 29.104 Cell Line Characterization Services Market for Asia-Pacific and Rest of the World: Forecasted Estimates (Till 2035), Conservative, Base and Optimistic Scenario (USD Million)
List of Figures
- Figure 2.1 Research Methodology: Research Assumptions
- Figure 2.2 Research Methodology: Project Methodology
- Figure 2.3 Research Methodology: Forecast Methodology
- Figure 2.4 Research Methodology: Robust Quality Control
- Figure 2.5 Research Methodology: Key Market Segmentations
- Figure 4.1 Executive Summary: Cell Line Development Service Providers Market Landscape
- Figure 4.2 Executive Summary: Cell Line Characterization Service Providers Market Landscape
- Figure 4.3 Executive Summary: Partnership and Collaborations
- Figure 4.4 Executive Summary: Recent Expansions
- Figure 4.5 Executive Summary: Cell line Development Service Providers Market Sizing and Opportunity Analysis
- Figure 4.6 Executive Summary: Cell line Characterization Service Providers Market Sizing and Opportunity Analysis
- Figure 5.1 Process of Cell Culturing
- Figure 5.2 Initiation of Cell Cultures
- Figure 5.3 Sources of Non-Mammalian Cell Lines
- Figure 5.4 Karyotype Preparation: Process Workflow
- Figure 5.5 DNA Hybridization: Process Workflow
- Figure 5.6 DNA Fingerprinting: Process Workflow
- Figure 5.7 Applications of Cell Lines
- Figure 5.8 Factors Affecting the Quality of Cell Lines
- Figure 5.9 Advantages and Risks Associated with Outsourcing of Cell Line Related Operations
- Figure 6.1 Cell Line Development Service Providers: Distribution by Year of Establishment
- Figure 6.2 Cell Line Development Service Providers: Distribution by Company Size
- Figure 6.3 Cell Line Development Service Providers: Distribution by Location of Headquarters
- Figure 6.4 Cell Line Development Service Providers: Distribution by Company Size and Location of Headquarters
- Figure 6.5 Cell Line Development Service Providers: Distribution by Source of Cell Line / Expression System Offered
- Figure 6.6 Cell Line Development Service Providers: Distribution by Gene Delivery Method Used
- Figure 6.7 Cell Line Development Service Providers: Distribution by Type of Transfection
- Figure 6.8 Cell Line Development Service Providers: Distribution by Availability of Serum-Free / Animal Component Free Culturing Capability
- Figure 6.9 Cell Line Development Service Providers: Distribution by Type of Cells Offered
- Figure 6.10 Cell Line Development Service Providers: Distribution by Application of Cell Line
- Figure 6.11 Cell Line Development Service Providers: Distribution by Additional Cell Line Related Services Offered
- Figure 6.12 Cell Line Development Service Providers: Distribution by Type of Cell Banking
- Figure 6.13 Cell Line Development Service Providers: Distribution by Availability of Integrated Cell Line Characterization Service
- Figure 7.1 Company Competitiveness Analysis: Cell Line Development Service Providers in North America
- Figure 7.2 Company Competitiveness Analysis: Cell Line Development Service Providers in Europe
- Figure 7.3 Company Competitiveness Analysis: Cell Line Development Service Providers in Asia-Pacific and rest of the world
- Figure 9.1 Cell Line Characterization Service Providers: Overall Market Landscape
- Figure 9.2 Cell Line Characterization Service Providers (Industry): Distribution by Year of Establishment
- Figure 9.3 Cell Line Characterization Service Providers (Industry): Distribution by Company Size
- Figure 9.4 Cell Line Characterization Service Providers (Industry): Distribution by Location of Headquarters
- Figure 9.5 Cell Line Characterization Service Providers (Industry): Distribution by Company Size and Location of Headquarters
- Figure 9.6 Cell Line Characterization Service Providers (Industry): Distribution by Cell Line Characterized
- Figure 9.7 Cell Line Characterization Service Providers (Industry): Distribution by Type of Cell Line Characterization Service Offered
- Figure 9.8 Cell Line Characterization Service Providers (Industry): Distribution by Type of Cell Line Identity / Stability Testing Service Offered
- Figure 9.9 Cell Line Characterization Service Providers (Industry): Distribution by Type of Cell Line Sterility / Biosafety Testing Service Offered
- Figure 9.10 Cell Line Characterization Service Providers (Non-Industry): Distribution by Location of Organization
- Figure 9.11 Cell Line Characterization Service Providers (Non-Industry): Distribution by Type of Cell Line Characterized
- Figure 9.12 Cell Line Characterization Service Providers (Non-Industry): Distribution by Type of Cell Line Characterization Service Offered
- Figure 9.13 Cell Line Characterization Service Providers (Non-Industry): Distribution by Genotyping Kit Used
- Figure 9.14 Cell Line Characterization Service Providers (Non-Industry): Distribution by Number of Loci Amplified
- Figure 10.1 Company Competitiveness Analysis: Cell Line Development Service Providers in North America
- Figure 10.2 Company Competitiveness Analysis: Cell Line Development Service Providers in Europe
- Figure 10.3 Company Competitiveness Analysis: Cell Line Development Service Providers in Asia-Pacific and rest of the world
- Figure 12.1 Partnerships and Collaborations: Cumulative Year-wise Trend, Since 2015
- Figure 12.2 Partnerships and Collaborations: Distribution by Type of Partnership
- Figure 12.3 Partnerships and Collaborations: Distribution by Year and Type of Partnership
- Figure 12.4 Partnerships and Collaborations: Distribution by Type of Partner
- Figure 12.5 Most Active Players: Distribution by Number of Partnerships
- Figure 12.6 Partnerships and Collaborations: Intracontinental and Intercontinental Deals
- Figure 12.7 Partnerships and Collaborations: International and Local Deals
- Figure 13.1 Recent Expansions: Cumulative Year-wise Trend, Since 2023
- Figure 13.2 Recent Expansions: Distribution by Type of Expansion
- Figure 13.3 Recent Expansions: Distribution by Year and Type of Expansion
- Figure 13.4 Recent Expansions: Distribution by Location of Headquarters and Company Size
- Figure 13.5 Recent Expansions: Distribution by Location of Expanded Facility
- Figure 13.6 Recent Expansions: Distribution by Type of Expansion and Location of Expanded Facility
- Figure 13.7 Recent Expansions: Distribution by Area of Expanded Facility (sq. ft.)
- Figure 13.8 Recent Expansions: Distribution by Purpose of Expansion
- Figure 13.9 Recent Expansions: Distribution by Geography
- Figure 13.10 Most Active Players: Distribution by Number of Recent Expansions
- Figure 14.1 American Type Culture Collection (ATCC): Service Portfolio
- Figure 14.2 Coriell Institute for Medical Research: Service Portfolio
- Figure 14.3 European Collection of Authenticated Cell Cultures (ECACC): Culture Collection
- Figure 14.4 European Collection of Authenticated Cell Cultures (ECACC): Service Portfolio
- Figure 14.5 Leibniz Institute DSMZ: Biological Material Collection
- Figure 14.6 National Centre for Cell Science (NCCS): Service Portfolio
- Figure 15.1 The International Council for Harmonisation Q5A (R1) Guidelines: Cell Bank Qualification
- Figure 15.2 The International Council for Harmonisation Q5D Guidelines: Cell Bank Characterization
- Figure 15.3 FDA Guidelines: Cell Bank Qualification
- Figure 15.4 US Pharmacopoeia (USP) 63: Mycoplasma Detection in Cell Banks
- Figure 15.5 Code for Federal Regulations (CFR) 610.18: Recommendations for Testing of Cell Lines
- Figure 16.1 Market Drivers
- Figure 16.2 Market Restrainers
- Figure 16.3 Market Opportunities
- Figure 16.4 Market Challenges
- Figure 17.1 Global Cell Line Development Services Market, Historical Trends (Since 2019) and Forecasted Estimates (Till 2035) (USD Million)
- Figure 17.2 Global Cell Line Development Services Market, 2023-2035, Optimistic Scenario (USD Million)
- Figure 17.3 Global Cell Line Development Services Market, 2023-2035, Conservative Scenario (USD Million)
- Figure 18.1 Cell Line Development Services Market: Distribution by Source of Cell Lines
- Figure 18.2 Cell Line Development Services Market for Mammalian Cell Lines: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035) (USD Million)
- Figure 18.3 Cell Line Development Services Market for Microbial Cell Lines: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035) (USD Million)
- Figure 18.4 Cell Line Development Services Market for Insect Cell Lines: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035) (USD Million)
- Figure 18.5 Cell Line Development Services Market for Other Cell Lines: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035) (USD Million)
- Figure 19.1 Cell Line Development Services Market: Distribution by Application of Cell Line
- Figure 19.2 Cell Line Development Services Market for Biomanufacturing: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035) (USD Million)
- Figure 19.3 Cell Line Development Services Market for Research and Development Operations: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035) (USD Million)
- Figure 20.2 Cell Line Development Services Market for Large Companies: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035) (USD Million)
- Figure 20.3 Cell Line Development Services Market for Mid-sized Companies: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035) (USD Million)
- Figure 20.4 Cell Line Development Services Market for Small Companies: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035) (USD Million)
- Figure 21.1 Cell Line Development Services Market: Distribution by Key Geographical Regions
- Figure 21.2 Cell Line Development Services Market for North America: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035) (USD Million)
- Figure 21.3 Cell Line Development Services Market for Europe: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035) (USD Million)
- Figure 21.4 Cell Line Development Services Market for Asia-Pacific and Rest of the World: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035) (USD Million)
- Figure 22.1 Global Cell Line Characterization Services Market, Historical Trends (Since 2019) and Forecasted Estimates (Till 2035) (USD Million)
- Figure 22.2 Global Cell Line Characterization Services Market, 2023-2035, Optimistic Scenario (USD Million)
- Figure 22.3 Global Cell Line Characterization Services Market, 2023-2035, Conservative Scenario (USD Million)
- Figure 23.1 Cell Line Characterization Services Market: Distribution by Source of Cell Line
- Figure 23.2 Cell Line Characterization Services Market for Mammalian Cell Lines: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035) (USD Million)
- Figure 23.3 Cell Line Characterization Services Market for Microbial Cell Lines: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035) (USD Million)
- Figure 23.4 Cell Line Characterization Services Market for Insect Cell Lines: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035) (USD Million)
- Figure 23.5 Cell Line Characterization Services Market for Other Cell Lines: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035) (USD Million)
- Figure 24.1 Cell Line Characterization Services Market: Distribution by Application of Cell Line
- Figure 24.2 Cell Line Characterization Services Market for Biomanufacturing: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035) (USD Million)
- Figure 24.3 Cell Line Characterization Services Market for Research and Characterization Operations: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035) (USD Million)
- Figure 25.1 Cell Line Characterization Services Market: Distribution by Company Size
- Figure 25.2 Cell Line Characterization Services Market for Large Companies: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035) (USD Million)
- Figure 25.3 Cell Line Characterization Services Market for Mid-sized Companies: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035) (USD Million)
- Figure 25.4 Cell Line Characterization Services Market for Small Companies: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035) (USD Million)
- Figure 26.1 Cell Line Characterization Services Market: Distribution by Key Geographical Regions
- Figure 26.2 Cell Line Characterization Services Market for North America: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035) (USD Million)
- Figure 26.3 Cell Line Characterization Services Market for Europe: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035) (USD Million)
- Figure 26.4 Cell Line Characterization Services Market for Asia-Pacific and Rest of the World: Historical Trends (Since 2019) and Forecasted Estimates (Till 2035) (USD Million)
- Figure 27.1 Concluding Remarks: Cell Line Development Service Providers Market Landscape
- Figure 27.2 Concluding Remarks: Cell Line Characterization Service Providers Market Landscape
- Figure 27.3 Concluding Remarks: Partnerships and Collaborations
- Figure 27.4 Concluding Remarks: Recent Expansions
- Figure 27.5 Concluding Remarks: Cell Line Development Service Providers Market Forecast and Opportunity Analysis
- Figure 27.6 Concluding Remarks: Cell Line Characterization Service Providers Market Forecast and Opportunity Analysis











