 |
市场调查报告书
商品编码
1771299
脂质CMO市场:产业趋势及全球预测 - 依脂质类型、公司规模、业务规模和主要地区Lipid CMO Market: Industry Trends and Global Forecasts - Distribution by Type of Lipid, Company Size, Scale Of Operation and Key Geographical Regions |
||||||
全球脂质CMO市场:概览
今年全球脂质CMO市场规模达24亿美元。预计预测期内,市场年复合成长率将达到 10.8%。
市场区隔包括根据以下参数进行的市场规模和机会分析:
脂质类型
- 脂质体/脂质奈米粒
- 磷脂
- 聚乙二醇化脂质
- 可离子化脂质(阳离子/阴离子脂质)
- 三酸甘油酯
- 鞘脂
- 中性脂质
- 其他
公司规模
- 小型
- 中型
- 大型/超大型
企业规模
- 临床前
- 临床试验
- 商业化
大型地区
- 北美
- 欧洲
- 亚太地区
- 中东和北非
- 拉丁美洲
- 世界其他地区
全球脂质CMO市场:成长与趋势
目前,约90%的在研候选药物和近40%的已核准药物面临与溶解度和渗透性相关的挑战。因此,在现代监管标准下,许多有前景的疗法由于生物利用度低而无法通过临床试验并进入市场。为了解决这些问题,生物製药产业的创新者设计了各种策略来改善药物化合物的理化性质和整体类药行为。在这些方法中,脂质奈米颗粒和其他能够提高生物膜渗透性的脂质基辅料引起了药物开发者的浓厚兴趣。事实上,基于mRNA的新型COVID-19疫苗已利用脂质奈米颗粒有效地将活性成分递送至人体内的标靶抗原呈现细胞。
此外,许多公司利用基于脂质的解决方案来重新配製现有的候选药物,以提高其生物利用度,这大幅推动了对脂质药物载体和辅料的需求。然而,某些医用脂质的生产,尤其是用于脂质体和脂质奈米颗粒製剂的生产,仍然高度复杂、资金密集且极具挑战性。因此,越来越多的製药公司将脂质的生产外包给专业机构。与医疗级脂质的合约生产组织(CMO)合作可带来许多益处,包括获得先进技术、扩大生产能力和提高营运灵活性。此外,预计在预测期内,对高品质脂质的需求不断成长将显着推动专业合约製造业的市场成长。
全球脂质CMO市场:关键洞察
本报告深入探讨了全球脂质CMO市场的现状,并识别了产业内的潜在成长机会。报告的主要调查结果包括:
- 目前,全球约有60家公司声称提供合约製造服务,以协助开发和生产用于治疗开发及其他相关产品的脂质。
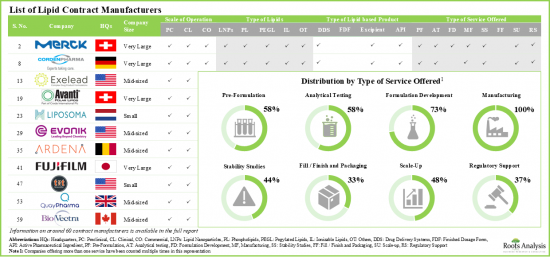
- 该行业中的一些CMO具备为不同类型的脂质产品提供广泛服务的必要能力,许多CMO拥有处理任何规模服务的能力。
- 近35%提供脂质DDS合约製造服务的公司声称拥有商业规模生产脂质体/LNP所需的能力和专业知识。
- 自2010年以来成立的利害关係人中,近40%有能力提供临床和商业规模的合约生产服务,其中约60%位于美国。
- 超大脂质体/LNP的代表性企业包括(依字母顺序排列)AMRI、CordenPharma、Evonik、Fresenius Kabi、Fujifilm、Merck和TTY Biopharm。
- 为了获得供应链能力并满足申办者不断变化的需求,脂质CMO公司关键地区建立业务。
- 为了追求竞争优势,企业投资或打算投资精力和资金来增强其现有的服务组合和合作产品。
- 最近,该行业的合作活动激增,其中大多数交易是为了从其他公司获得独特能力而签署的。
- 由于全球对用于生产基于 mRNA 的COVID-19 候选疫苗的脂质奈米颗粒的需求激增,脂质 CMO 行业的交易量激增。
- 签署的合作关係中,近 50%是製造协议。此外,约 65%的协议是为製造基于脂质的药物递送系统或辅料而签署的。
- 近 75%的合作关係以北美为中心。与北美公司签署协议的公司包括 ABITEC、AMRI、Catalent 和 Precision NanoSystems。
- 为了满足日益成长的脂质需求,CMO/CDMO近年来进行了大规模扩张,其中大部分在美国设立了生产基地。
- 近年来,脂质製剂已成为提高药物生物利用度的一种流行方法,近55家公司为此开发各种脂质相关技术。
- 该市场的供应端主要由中型和大型CMO驱动。值得注意的是,目前有近65%的产能位于北美生产工厂。
- 从中长期来看,预计脂质製剂开发商将继续将生产营运外包给CMO/CDMO,使该服务市场以超过10.8%的年复合成长率成长。
脂质CMO市场参与者
- Avanti Polar Lipids
- Creative Biolabs
- Exelead
- FormuMax Scientific
- T&T Scientific
- Ardena
- Corden Pharma
- Evonik
- Fresenius Kabi
- Merck KGaA
- Fujifilm
- Nagase Medicals
- Nippon Fine Chemical
- TTY Biopharm
- VCARE Bio Labs
本报告调查全球脂质CMO市场提供市场概述,以及依脂质类型、公司规模、业务规模和地区的趋势,和参与市场的公司简介。
目录
第1章 引言
第2章 执行摘要
第3章 导论
- 章节概述
- 脂质简介
- 脂质在製药业的应用
- 脂质製造面临的挑战
- 脂质製造外包的需求
- 结论
第4章 竞争格局
- 章节概述
- 脂质CMO参与者:市场格局
第5章 竞争格局分析
- 章节概述
- 假设和关键参数
- 研究方法
- 脂质CMO 厂商:竞争格局分析
第6章 公司简介:北美脂质 CMO 厂商
- 章节概述
- Avanti Polar Lipids
- Creative Biolabs
- Exelead
- FormuMax Scientific
- T&T Scientific
第7章 公司简介:欧洲脂质 CMO 厂商
- 章节概述
- Ardena
- CordenPharma
- Evonik
- Fresenius Kabi
- Merck KGaA
第8章 公司简介:亚太脂质 CMO厂商
- 章节概述
- 富士软片
- 长濑医疗
- 日本精细化学
- TTY 生物製药
- VCARE 生物实验室
第9章 合作伙伴关係与合作
- 章节概述
- 合作模式
- 脂质CMO市场:合作伙伴关係与合作
第10章 近期扩张
第11章 产能分析
- 章节概述
- 关键假设与研究方法
- 脂质合约製造:全球装置容量(百万公升)
- 结论
第12章 自主或外购决策架构
- 章节概述
- 假设和参数定义
- 脂质合约製造:自主或外购决策
- 结论
第13章 市场预测与机会分析
- 章节概述
- 预测研究方法与关键假设
- 2035年全球脂质CMO市场
- 脂质CMO市场(2035年):依脂质类型
- 脂质CMO市场(2035年):依公司规模
- 脂质CMO市场(2035年):依公司规模
- 脂质CMO市场(2035年):依公司规模
- 脂质CMO市场(2035年):依区域
第14章 案例研究:脂质及脂质製剂技术的应用
- 章节概述
- 生物药学分类系统
- 脂质製剂作为药物传递系统
- 脂质製剂技术开发者
第15章 结论
第16章 高层洞察
第17章 附录1:表格资料
第18章 附录2:公司与组织清单
GLOBAL LIPID CMO MARKET: OVERVIEW
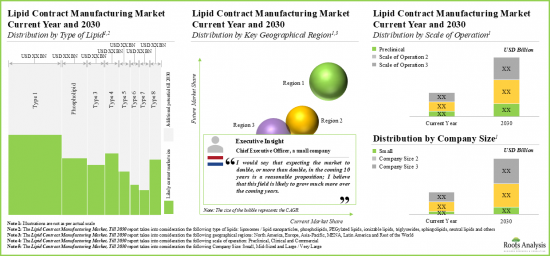
As per Roots Analysis, the global lipid CMO market valued at USD 2.4 billion in the current year is expected to grow at a CAGR of 10.8% during the forecast period.
The market sizing and opportunity analysis has been segmented across the following parameters:
Type of Lipid
- Liposomes / Lipid Nanoparticles
- Phospholipids
- Pegylated Lipids
- Ionizable Lipids (Cationic / Anionic Lipids)
- Triglycerides
- Sphingolipids
- Neutral Lipids
- Others
Company Size
- Small
- Mid-Sized
- Large / Very Large
Scale Of Operation
- Preclinical
- Clinical
- Commercial
Key Geographical Regions
- North America
- Europe
- Asia-Pacific
- Middle East and North Africa
- Latin America
- Rest of the World
GLOBAL LIPID CMO MARKET: GROWTH AND TRENDS
Currently, ~ 90% of drug candidates in the development stage and nearly 40% of approved pharmaceutical products face challenges related to solubility and permeability. As a result, under modern regulatory standards, many promising therapeutic leads fail to progress through clinical trials and reach the market due to poor bioavailability. To address these issues, innovators in the biopharmaceutical industry have devised various strategies to enhance the physicochemical properties and overall drug-like behavior of pharmaceutical compounds. Among these approaches, lipid nanoparticles and other lipid-based excipients, which improve permeability across biological membranes have attracted significant interest from drug developers. In fact, the novel mRNA-based COVID-19 vaccines utilized lipid nanoparticles to effectively deliver their active ingredients to target antigen-presenting cells within the human body.

Furthermore, numerous companies are leveraging lipid-based solutions to reformulate existing drug candidates and enhance their bioavailability, driving significant growth in the demand for lipid drug carriers and excipients. However, manufacturing certain medically relevant lipids, particularly those used in liposome and lipid nanoparticle formulations remains highly complex, capital-intensive, and challenging. Consequently, an increasing number of pharmaceutical companies are opting to outsource lipid manufacturing to specialized providers. Partnering with contract manufacturing organizations (CMOs) for medical-grade lipids offers several advantages, including access to advanced technologies, greater production capacity and enhanced operational flexibility. Moreover, we anticipate that the growing demand for high-quality lipids will significantly propel market growth in the specialty contract manufacturing sector over the forecast period.
GLOBAL LIPID CMO MARKET: KEY INSIGHTS
The report delves into the current state of global lipid CMO market and identifies potential growth opportunities within industry. Some key findings from the report include:
- Presently, around 60 players, across the world, claim to provide contract manufacturing services to support the development and production of lipids for use in therapeutic development, as well as other associated products.
- Several CMOs engaged in this industry have the required capabilities to offer a range of services for different types of lipid-based products; many CMOs have the capacity to operate across all scales.
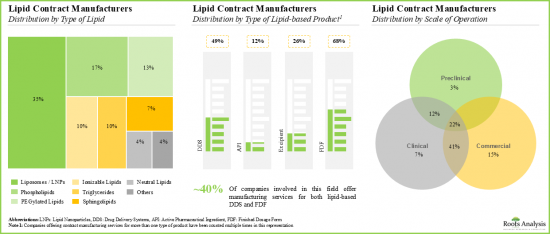
- Close to 35% of players offering contract manufacturing services for lipid-based DDS claim to have the required capabilities and expertise to manufacture liposomes / LNPs at the commercial scale.

- Nearly 40% of the stakeholders established post-2010 have the capability to offer contract manufacturing services at both clinical and commercial scales; around 60% of these players are based in the US.
- Prominent examples of very large players offering liposomes / LNPs include (in alphabetical order) AMRI, CordenPharma, Evonik, Fresenius Kabi, Fujifilm, Merck and TTY Biopharm.
- In order to acquire supply chain competencies and meet the evolving needs of sponsors, lipid contract manufacturers have established a presence in key geographical regions.
- In pursuit of competitive edge, companies have made / revealed intentions to make investments, in terms of both effort and capital, to augment their existing service portfolios and affiliated offerings.
- Recently, there has been a surge in partnership activity within this industry; most deals were inked for the purpose of acquiring the proprietary capabilities of other players
- The lipid CMO industry witnessed a surge in the number of deals owing to the sudden increase in demand for lipid nanoparticles for the production of mRNA-based COVID-19 vaccine candidates, globally.
- Close to 50% of the partnerships inked were manufacturing agreements. Further, around 65% of these agreements were signed for the production of either lipid-based drug delivery systems or excipients.
- Nearly 75% of the partnership activity is centered in North America. Examples of firms that have signed deals within players based in North America, include ABITEC, AMRI, Catalent and Precision NanoSystems.
- To keep pace with the growing demand for lipids, CMOs / CDMOs have undertaken a number of expansion initiatives in the recent past; the maximum number of such instances involved facilities based in the US.
- Lipid-based formulations have emerged as a popular approach for improving drug bioavailability over the past years; close to 55 companies have developed various lipid-related technologies for this purpose.
- The supply side of this market is primarily driven by mid-to-large sized CMOs; interestingly, close to 65% of the currently available capacity is installed in production plants located in North America.

- In the mid-long term, we expect lipid-based formulation developers to continue outsourcing their manufacturing operations to CMOs / CDMOs, thereby, enabling this services market to grow at a CAGR of more than 10.8%.
Example Players in the Lipid CMO Market
- Avanti Polar Lipids
- Creative Biolabs
- Exelead
- FormuMax Scientific
- T&T Scientific
- Ardena
- Corden Pharma
- Evonik
- Fresenius Kabi
- Merck KGaA
- Fujifilm
- Nagase Medicals
- Nippon Fine Chemical
- TTY Biopharm
- VCARE Bio Labs
PRIMARY RESEARCH OVERVIEW
The opinions and insights presented in this study were influenced by discussions conducted with multiple stakeholders. The research report features detailed transcripts of interviews conducted with the following industry stakeholders:
- Founder and Chief Executive Officer, Company A
- Associate Director, Formulation Development, Company B
- Acting Manager, Biotech Process Development and Director, Process Development - Chemistry, Company C
GLOBAL LIPID CMO MARKET
- Market Sizing and Opportunity Analysis: The report features an in-depth analysis of the global lipid CMO market, focusing on key market segments, including [A] type of lipid, [B] company size, [C] scale of operation and [D] key geographical regions.
- Market Landscape: A comprehensive evaluation of companies offering contract services for the manufacturing of lipids, considering various parameters, such as [A] year of establishment, [B] company size, [C] scale of operation, [D] location of headquarters, [E] location of manufacturing facilities, [F] type of product, [G] type of service(s) offered and [H] type of lipid manufactured.
- Company Competitiveness Analysis: An insightful competitive analysis of the lipid manufacturers, examining factors, such as [A] supplier power and [B] service strength.
- Company Profiles: In-depth profiles of companies that offer various lipid manufacturing services, across North America, Europe and Asia-Pacific, focusing on [A] company overview, [B] financial performance (if available), [C] service portfolio and [D] recent developments and an informed future outlook.
- Partnerships and Collaborations: An in-depth analysis of the deals inked by stakeholders in this domain, based on several parameters, such as [A] year of partnership, [B] type of partnership, [C] type of focus area, [D] most active players (in terms of the number of partnerships signed) and [E] geographical distribution of partnership activity.
- Recent Expansions: A detailed analysis of the recent expansions, based on several relevant parameters, including [A] year of expansion, [B] type of expansion, [C] scale of operation, [D] type of product, [E] amount invested, [F] company size, [G] location of headquarters, [H] geographical location of the expanded facility, [I] most active players and [J] geographical distribution of the expansion activity.
- Capacity Analysis: A comprehensive analysis of global installed capacity for lipids, based on several relevant parameters, such as [A] company size, [B] scale of operation and [C] key geographical regions.
- Make Versus Buy Decision Making Framework: A detailed analysis, highlighting the various factors that need to be taken into consideration by lipid developers while deciding whether to manufacture their respective products in-house or engage the services of a CMO.
- Case study: An elaborate discussion on the wide adoption of lipids as drug delivery systems in mRNA vaccines.
KEY QUESTIONS ANSWERED IN THIS REPORT
- How many companies are currently engaged in this market?
- Which are the leading companies in this market?
- What factors are likely to influence the evolution of this market?
- What is the current and future market size?
- What is the CAGR of this market?
- How is the current and future market opportunity likely to be distributed across key market segments?
REASONS TO BUY THIS REPORT
- The report provides a comprehensive market analysis, offering detailed revenue projections of the overall market and its specific sub-segments. This information is valuable to both established market leaders and emerging entrants.
- Stakeholders can leverage the report to gain a deeper understanding of the competitive dynamics within the market. By analyzing the competitive landscape, businesses can make informed decisions to optimize their market positioning and develop effective go-to-market strategies.
- The report offers stakeholders a comprehensive overview of the market, including key drivers, barriers, opportunities, and challenges. This information empowers stakeholders to stay abreast of market trends and make data-driven decisions to capitalize on growth prospects.
ADDITIONAL BENEFITS
- Complimentary PPT Insights Packs
- Complimentary Excel Data Packs for all Analytical Modules in the Report
- 15% Free Content Customization
- Detailed Report Walkthrough Session with Research Team
- Free Updated report if the report is 6-12 months old or older
TABLE OF CONTENTS
1. PREFACE
- 1.1. Scope of the Report
- 1.2. Research Methodology
- 1.2.1. Research Assumptions
- 1.2.2. Project Methodology
- 1.2.3. Forecast Methodology
- 1.2.4. Robust Quality Control
- 1.2.5. Key Considerations
- 1.2.5.1. Demographics
- 1.2.5.2. Economic Factors
- 1.2.5.3. Government Regulations
- 1.2.5.4. Supply Chain
- 1.2.5.5. COVID Impact / Related Factors
- 1.2.5.6. Market Access
- 1.2.5.7. Healthcare Policies
- 1.2.5.8. Industry Consolidation
- 1.3 Key Questions Answered
- 1.4. Chapter Outlines
2. EXECUTIVE SUMMARY
3. INTRODUCTION
- 3.1. Chapter Overview
- 3.2. Introduction to Lipids
- 3.2.1. Types of Lipids
- 3.3. Applications of Lipids in Pharmaceutical Industry
- 3.4. Challenges Associated with Lipid Manufacturing
- 3.5. Need for Outsourcing Lipid Manufacturing
- 3.6. Concluding Remarks
4. COMPETITIVE LANDSCAPE
- 4.1. Chapter Overview
- 4.2. Lipid Contract Manufacturers: Overall Market Landscape
- 4.2.1. Analysis by Year of Establishment
- 4.2.2. Analysis by Company Size
- 4.2.3. Analysis by Scale of Operation
- 4.2.4. Analysis by Location of Headquarters
- 4.2.5. Analysis by Location of Lipid Manufacturing Facilities
- 4.2.6. Analysis by Type of Product
- 4.2.7. Analysis by Service(s) Offered
- 4.2.8. Analysis by Types of Lipids Manufactured
- 4.2.8.1. Analysis by Types of Lipids Manufactured and Company Size
- 4.2.8.2. Analysis by Types of Lipids Manufactured and Scale of Operation
- 4.2.8.3. Analysis by Types of Lipids Manufactured and Location of Headquarters
5. COMPANY COMPETITIVENESS ANALYSIS
- 5.1. Chapter Overview
- 5.2. Assumptions and Key Parameters
- 5.3. Methodology
- 5.4. Lipid Contract Manufacturers: Company Competitiveness Analysis
- 5.4.1. Company Competitiveness Analysis: Lipid Contract Manufacturers in North America
- 5.4.2. Company Competitiveness Analysis: Lipid Contract Manufacturers in Europe
- 5.4.3. Company Competitiveness Analysis: Lipid Contract Manufacturers in Asia-Pacific
6. COMPANY PROFILES: LIPID CONTRACT MANUFACTURERS IN NORTH AMERICA
- 6.1. Chapter Overview
- 6.2. Avanti Polar Lipids
- 6.2.1. Company Overview
- 6.2.2. Lipid Manufacturing Service Offerings
- 6.2.3. Manufacturing Facilities
- 6.2.4. Recent Developments and Future Outlook
- 6.3. Creative Biolabs
- 6.3.1. Company Overview
- 6.3.2. Lipid Manufacturing Service Offerings
- 6.3.3. Manufacturing Facilities
- 6.3.4. Recent Developments and Future Outlook
- 6.4. Exelead
- 6.4.1. Company Overview
- 6.4.2. Lipid Manufacturing Service Offerings
- 6.4.3. Manufacturing Facilities
- 6.4.4. Recent Developments and Future Outlook
- 6.5. FormuMax Scientific
- 6.5.1. Company Overview
- 6.5.2. Lipid Manufacturing Service Offerings
- 6.5.3. Manufacturing Facilities
- 6.5.4. Recent Developments and Future Outlook
- 6.6. T&T Scientific
- 6.6.1. Company Overview
- 6.6.2. Lipid Manufacturing Service Offerings
- 6.6.3. Manufacturing Facilities
- 6.6.4. Recent Developments and Future Outlook
7. COMPANY PROFILES: LIPID CONTRACT MANUFACTURERS IN EUROPE
- 7.1. Chapter Overview
- 7.2. Ardena
- 7.2.1. Company Overview
- 7.2.2. Lipid Manufacturing Service Offerings
- 7.2.3. Manufacturing Facilities
- 7.2.4. Recent Developments and Future Outlook
- 7.3. CordenPharma
- 7.3.1. Company Overview
- 7.3.2. Lipid Manufacturing Service Offerings
- 7.3.3. Manufacturing Facilities
- 7.3.4. Recent Developments and Future Outlook
- 7.4. Evonik
- 7.4.1. Company Overview
- 7.4.2. Lipid Manufacturing Service Offerings
- 7.4.3. Manufacturing Facilities
- 7.4.4. Recent Developments and Future Outlook
- 7.5. Fresenius Kabi
- 7.5.1. Company Overview
- 7.5.2. Lipid Manufacturing Service Offerings
- 7.5.3. Manufacturing Facilities
- 7.5.4. Recent Developments and Future Outlook
- 7.6. Merck KGaA
- 7.6.1. Company Overview
- 7.6.2. Lipid Manufacturing Service Offerings
- 7.6.3. Manufacturing Facilities
- 7.6.4. Recent Developments and Future Outlook
8. COMPANY PROFILES: LIPID CONTRACT MANUFACTURERS IN ASIA-PACIFIC
- 8.1. Chapter Overview
- 8.2. Fujifilm
- 8.2.1. Company Overview
- 8.2.2. Lipid Manufacturing Service Offerings
- 8.2.3. Manufacturing Facilities
- 8.2.4. Recent Developments and Future Outlook
- 8.3. Nagase Medicals
- 8.3.1. Company Overview
- 8.3.2. Lipid Manufacturing Service Offerings
- 8.3.3. Manufacturing Facilities
- 8.3.4. Recent Developments and Future Outlook
- 8.4. Nippon Fine Chemical
- 8.4.1. Company Overview
- 8.4.2. Lipid Manufacturing Service Offerings
- 8.4.3. Manufacturing Facilities
- 8.4.4. Recent Developments and Future Outlook
- 8.5. TTY Biopharm
- 8.5.1. Company Overview
- 8.5.2. Lipid Manufacturing Service Offerings
- 8.5.3. Manufacturing Facilities
- 8.5.4. Recent Developments and Future Outlook
- 8.6. VCARE Bio Labs
- 8.6.1. Company Overview
- 8.6.2. Lipid Manufacturing Service Offerings
- 8.6.3. Manufacturing Facilities
- 8.6.4. Recent Developments and Future Outlook
9. PARTNERSHIPS AND COLLABORATIONS
- 9.1. Chapter Overview
- 9.2. Partnership Models
- 9.3. Lipid Contract Manufacturing Market: Partnerships and Collaborations
- 9.3.1. Analysis by Year of Partnership
- 9.3.2. Analysis by Type of Partnership
- 9.3.3. Analysis by Type of Product
- 9.3.4. Analysis by Type of Focus Area
- 9.3.5. Most Active Players: Analysis by Number of Partnerships
- 9.3.6. Geographical Analysis
- 9.3.6.1. Region-wise Distribution
- 9.3.6.2. Country-wise Distribution
10. RECENT EXPANSIONS
- 10.1. Chapter Overview
- 10.2. Lipid Contract Manufacturing Market: Recent Expansions
- 10.2.1. Analysis by Year of Expansion
- 10.2.2. Analysis by Type of Expansion
- 10.2.3. Analysis by Type of Expansion and Scale of Operation
- 10.2.4. Analysis by Type of Product
- 10.2.5. Analysis by Amount Invested
- 10.2.6. Analysis by Company Size and Location of Headquarters
- 10.2.7. Analysis by Location of Expanded Facility
- 10.2.8. Most Active Players: Analysis by Number of Recent Expansions
- 10.2.9. Geographical Analysis
- 10.2.9.1. Region-wise Distribution
- 10.2.9.2. Country-wise Distribution
11. CAPACITY ANALYSIS
- 11.1. Chapter Overview
- 11.2. Key Assumptions and Methodology
- 11.3. Lipid Contract Manufacturing: Installed Global Capacity (Million Liters)
- 11.3.1. Analysis by Company Size
- 11.3.2. Analysis by Scale of Operation
- 11.3.3. Analysis by Location of Manufacturing Facility
- 11.4. Concluding Remarks
12. MAKE VERSUS BUY DECISION MAKING FRAMEWORK
- 12.1. Chapter Overview
- 12.2. Assumptions and Parameter Definitions
- 12.3. Lipid Contract Manufacturing: Make Versus Buy Decision Making
- 12.3.1. Scenario 1
- 12.3.2. Scenario 2
- 12.3.3. Scenario 3
- 12.3.4. Scenario 4
- 12.4. Concluding Remarks
13. MARKET FORECAST AND OPPORTUNITY ANALYSIS
- 13.1. Chapter Overview
- 13.2. Forecast Methodology and Key Assumptions
- 13.3. Global Lipid Contract Manufacturing Market, Till 2035
- 13.4. Lipid Contract Manufacturing Market, Till 2035: Distribution by Type of Lipid
- 13.5. Lipid Contract Manufacturing Market, Till 2035: Distribution by Company Size
- 13.6. Lipid Contract Manufacturing Market, Till 2035: Distribution by Scale of Operation
- 13.7. Lipid Contract Manufacturing Market, Till 2035: Distribution by Region
- 13.7.1. Lipid Contract Manufacturing Market in North America, Till 2035
- 13.7.2. Lipid Contract Manufacturing Market in Europe, Till 2035
- 13.7.3. Lipid Contract Manufacturing Market in Asia-Pacific, Till 2035
- 13.7.4. Lipid Contract Manufacturing Market in MENA, Till 2035
- 13.7.5. Lipid Contract Manufacturing Market in Latin America, Till 2035
- 13.7.6. Lipid Contract Manufacturing Market in Rest of the World, Till 2035
14. CASE STUDY: APPLICATIONS OF LIPIDS AND LIPID FORMULATION TECHNOLOGIES
- 14.1. Chapter Overview
- 14.2. Biopharmaceutical Drug Classification System
- 14.3. Lipid-based Formulations as Drug Delivery Systems
- 14.3.1. Role of Lipids in mRNA Vaccines
- 14.3.2. Recent Developments in Lipid Nanoparticles
- 14.4. Lipid-based Formulation Technology Developers
- 14.4.1. Analysis by Year of Establishment
- 14.4.2. Analysis by Company Size
- 14.4.3. Analysis by Location of Headquarters
15. CONCLUDING REMARKS
16. EXECUTIVE INSIGHTS
- 16.1. Chapter Overview
- 16.2. Company A
- 16.2.1. Company Snapshot
- 16.2.2. Interview Transcript: Founder and CEO
- 16.3. Company B
- 16.3.1. Company Snapshot
- 16.3.2. Interview Transcript: Associate Director, Formulation Development
- 16.4. Company C
- 16.4.1. Company Snapshot
- 16.4.2. Interview Transcript: Acting Manager, Biotech Process Development and Director, Process Development - Chemistry
17. APPENDIX 1: TABULATED DATA
18. APPENDIX 2: LIST OF COMPANIES AND ORGANIZATIONS
List of Tables
- Table 4.1 List of Lipid Contract Manufacturers
- Table 4.2 Lipid Contract Manufacturers: Information on Number and Location of Manufacturing Facilities
- Table 4.3 Lipid Contract Manufacturers: Information on Type of Product and Scale of Operation
- Table 4.4 Lipid Contract Manufacturers: Information on Service(s) Offered
- Table 4.5 Lipid Contract Manufacturers: Information on Types of Lipids Manufactured
- Table 6.1 Lipid Contract Manufacturers: List of Profiled Companies (North America)
- Table 6.2 Avanti Polar Lipids: Company Snapshot
- Table 6.3 Avanti Polar Lipids: Lipid-related Service Offerings
- Table 6.4 Avanti Polar Lipids: Information on Manufacturing Facility
- Table 6.5 Avanti Polar Lipids: Recent Developments and Future Outlook
- Table 6.6 Creative Biolabs: Company Snapshot
- Table 6.7 Creative Biolabs: Lipid-related Service Offerings
- Table 6.8 Creative Biolabs: Information on Manufacturing Facility
- Table 6.9 Exelead: Company Snapshot
- Table 6.10 Exelead: Lipid-related Service Offerings
- Table 6.11 Exelead: Information on Manufacturing Facility
- Table 6.12 Exelead: Recent Developments and Future Outlook
- Table 6.13 FormuMax Scientific: Company Snapshot
- Table 6.14 FormuMax Scientific: Lipid-related Service Offerings
- Table 6.15 FormuMax Scientific: Information on Manufacturing Facility
- Table 6.16 T&T Scientific: Company Snapshot
- Table 6.17 T&T Scientific: Lipid-related Service Offerings
- Table 6.18 T&T Scientific: Information on Manufacturing Facility
- Table 6.19 T&T Scientific: Recent Developments and Future Outlook
- Table 7.1 Lipid Contract Manufacturers: List of Profiled Companies (Europe)
- Table 7.2 Ardena: Company Snapshot
- Table 7.3 Ardena: Lipid-related Service Offerings
- Table 7.4 Ardena: Information on Manufacturing Facility
- Table 7.5 Ardena: Recent Developments and Future Outlook
- Table 7.6 CordenPharma: Company Snapshot
- Table 7.7 CordenPharma: Lipid-related Service Offerings
- Table 7.8 CordenPharma: Information on Manufacturing Facility
- Table 7.9 CordenPharma: Recent Developments and Future Outlook
- Table 7.10 Evonik: Company Snapshot
- Table 7.11 Evonik: Lipid-related Service Offerings
- Table 7.12 Evonik: Information on Manufacturing Facility
- Table 7.13 Evonik: Recent Developments and Future Outlook
- Table 7.14 Fresenius Kabi: Company Snapshot
- Table 7.15 Fresenius Kabi: Lipid-related Service Offerings
- Table 7.16 Fresenius Kabi: Information on Manufacturing Facility
- Table 7.17 Fresenius Kabi: Recent Developments and Future Outlook
- Table 7.18 Merck KGaA: Company Snapshot
- Table 7.19 Merck KGaA: Lipid-related Service Offerings
- Table 7.20 Merck KGaA: Information on Manufacturing Facility
- Table 7.21 Merck KGaA: Recent Developments and Future Outlook
- Table 8.1 Lipid Contract Manufacturers: List of Profiled Companies (Asia-Pacific)
- Table 8.2 Fujifilm: Company Snapshot
- Table 8.3 Fujifilm: Lipid-related Service Offerings
- Table 8.4 Fujifilm: Information on Manufacturing Facility
- Table 8.5 Fujifilm: Recent Developments and Future Outlook
- Table 8.6 Nagase Medicals: Company Snapshot
- Table 8.7 Nagase Medicals: Lipid-related Service Offerings
- Table 8.8 Nagase Medicals: Information on Manufacturing Facility
- Table 8.9 Nagase Medicals: Recent Developments and Future Outlook
- Table 8.10 Nippon Fine Chemical: Company Snapshot
- Table 8.11 Nippon Fine Chemical: Lipid-related Service Offerings
- Table 8.12 Nippon Fine Chemical: Information on Manufacturing Facility
- Table 8.13 TTY Biopharm: Company Snapshot
- Table 8.14 TTY Biopharm: Lipid-related Service Offerings
- Table 8.15 TTY Biopharm: Information on Manufacturing Facility
- Table 8.16 TTY Biopharm: Recent Developments and Future Outlook
- Table 8.17 VCARE Bio Labs: Company Snapshot
- Table 8.18 VCARE Bio Labs: Lipid-related Service Offerings
- Table 9.1 Lipid Contract Manufacturers: List of Partnerships and Collaborations, Since 2016
- Table 10.1 Lipid Contract Manufacturers: Recent Expansions, Since 2016
- Table 14.1 Lipid Formulation Technologies: List of Developers
- Table 14.2 List of Approved Lipid-based Drugs
- Table 17.1 Lipid Contract Manufacturers: Distribution by Year of Establishment
- Table 17.2 Lipid Contract Manufacturers: Distribution by Company Size
- Table 17.3 Lipid Contract Manufacturers: Distribution by Scale of Operation
- Table 17.4 Lipid Contract Manufacturers: Distribution by Location of Headquarters (Region-wise)
- Table 17.5 Lipid Contract Manufacturers: Distribution by Location of Headquarters (Country-wise)
- Table 17.6 Lipid Contract Manufacturers: Distribution by Company Size and Location of Headquarters
- Table 17.7 Lipid Contract Manufacturers: Distribution by Location of Lipid Manufacturing Facilities (Region-wise)
- Table 17.8 Lipid Contract Manufacturers: Distribution by Location of Lipid Manufacturing Facilities (Country-wise)
- Table 17.9 Lipid Contract Manufacturers: Distribution by Type of Product
- Table 17.10 Lipid Contract Manufacturers: Distribution by Service(s) Offered
- Table 17.11 Lipid Contract Manufacturers: Distribution by Type of Product and Service(s) Offered
- Table 17.12 Lipid Contract Manufacturers: Distribution by Service(s) Offered and Location of Headquarters
- Table 17.13 Lipid Contract Manufacturers: Distribution by Types of Lipids Manufactured
- Table 17.14 Lipid Contract Manufacturers: Distribution by Types of Lipids Manufactured and Company Size
- Table 17.15 Lipid Contract Manufacturers: Distribution by Types of Lipids Manufactured and Scale of Operation
- Table 17.16 Lipid Contract Manufacturers: Distribution by Types of Lipids Manufactured and Location of Headquarters
- Table 17.17 Partnerships and Collaborations: Cumulative Year-wise Trend, Since 2016
- Table 17.18 Partnerships and Collaborations: Distribution by Type of Partnership
- Table 17.19 Partnerships and Collaborations: Year-wise Trend by Type of Partnership
- Table 17.20 Partnerships and Collaborations: Distribution by Type of Product
- Table 17.21 Partnerships and Collaborations: Year-wise Trend by Type of Product, Since 2016
- Table 17.22 Partnerships and Collaborations: Distribution by Type of Focus Area
- Table 17.23 Most Active Players: Distribution by Number of Partnerships
- Table 17.24 Partnerships and Collaborations: Distribution by Region (Continent-wise)
- Table 17.25 Partnerships and Collaborations: Distribution by Region (Country-wise)
- Table 17.26 Recent Expansions: Cumulative Year-wise Trend, Since 2016
- Table 17.27 Recent Expansions: Distribution by Type of Expansion
- Table 17.28 Recent Expansions: Distribution by Type of Expansion and Scale of Operation
- Table 17.29 Recent Expansions: Distribution by Type of Product
- Table 17.30 Recent Expansions: Distribution by Type of Expansion and Type of Product
- Table 17.31 Recent Expansions: Distribution by Amount Invested (USD Million)
- Table 17.32 Recent Expansions: Distribution by Company Size and Location of Headquarters
- Table 17.33 Recent Expansions: Distribution by Location of Expanded Facility
- Table 17.34 Recent Expansions: Distribution by Type of Expansion and Location of Expanded Facility
- Table 17.35 Most Active Players: Distribution by Number of Recent Expansions
- Table 17.36 Recent Expansions: Distribution by Year of Expansion and Region
- Table 17.37 Recent Expansions: Distribution by Region (Country-wise)
- Table 17.38 Lipid Contract Manufacturing Capacity: Distribution by Company Size
- Table 17.39 Lipid Contract Manufacturing Capacity: Distribution by Scale of Operation
- Table 17.40 Lipid Contract Manufacturing Capacity: Distribution by Location of Manufacturing Facility
- Table 17.41 Global Lipid Contract Manufacturing Market, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
- Table 17.42 Lipid Contract Manufacturing Market, Conservative, Base and Optimistic Scenarios, Till 2035: Distribution by Type of Product (USD Billion)
- Table 17.43 Lipid Contract Manufacturing Market, Conservative, Base and Optimistic Scenarios, Till 2035: Distribution by Company Size (USD Billion)
- Table 17.44 Lipid Contract Manufacturing Market, Conservative, Base and Optimistic Scenarios, Till 2035: Distribution by Scale of Operation (USD Billion)
- Table 17.45 Lipid Contract Manufacturing Market in North America, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
- Table 17.46 Lipid Contract Manufacturing Market in Europe, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
- Table 17.47 Lipid Contract Manufacturing Market in Asia-Pacific, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
- Table 17.48 Lipid Contract Manufacturing Market in MENA, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
- Table 17.49 Lipid Contract Manufacturing Market in Latin America, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
- Table 17.50 Lipid Contract Manufacturing Market in Rest of the World, Conservative, Base and Optimistic Scenarios, Till 2035 (USD Billion)
List of Figures
- Figure 3.1 Applications of Lipid-based Formulations
- Figure 3.2 Classification of Lipids
- Figure 4.1 Lipid Contract Manufacturers: Distribution by Year of Establishment
- Figure 4.2 Lipid Contract Manufacturers: Distribution by Company Size
- Figure 4.3 Lipid Contract Manufacturers: Distribution by Scale of Operation
- Figure 4.4 Lipid Contract Manufacturers: Distribution by Location of Headquarters (Region-wise)
- Figure 4.5 Lipid Contract Manufacturers: Distribution by Location of Headquarters (Country-wise)
- Figure 4.6 Lipid Contract Manufacturers: Distribution by Company Size and Location of Headquarters
- Figure 4.7 Lipid Contract Manufacturers: Distribution by Location of Lipid Manufacturing Facilities (Region-wise)
- Figure 4.8 Lipid Contract Manufacturers: Distribution by Location of Lipid Manufacturing Facilities (Country-wise)
- Figure 4.9 Lipid Contract Manufacturers: Distribution by Type of Product
- Figure 4.10 Lipid Contract Manufacturers: Distribution by Service(s) Offered
- Figure 4.11 Lipid Contract Manufacturers: Distribution by Type of Product and Service(s) Offered
- Figure 4.12 Lipid Contract Manufacturers: Distribution by Service(s) Offered and Location of Headquarters
- Figure 4.13 Lipid Contract Manufacturers: Distribution by Types of Lipids Manufactured
- Figure 4.14 Lipid Contract Manufacturers: Distribution by Types of Lipids Manufactured and Company Size
- Figure 4.15 Lipid Contract Manufacturers: Distribution by Types of Lipids Manufactured and Scale of Operation
- Figure 4.16 Lipid Contract Manufacturers: Distribution by Types of Lipids Manufactured and Location of Headquarters
- Figure 5.1 Company Competitiveness Analysis: Lipid Contract Manufacturers in North America
- Figure 5.2 Company Competitiveness Analysis: Lipid Contract Manufacturers in Europe
- Figure 5.3 Company Competitiveness Analysis: Lipid Contract Manufacturers in Asia-Pacific
- Figure 9.1 Partnerships and Collaborations: Cumulative Year-wise Trend, Since 2016
- Figure 9.2 Partnerships and Collaborations: Distribution by Type of Partnership
- Figure 9.3 Partnerships and Collaborations: Year-wise Trend by Type of Partnership
- Figure 9.4 Partnerships and Collaborations: Distribution by Type of Product
- Figure 9.5 Partnerships and Collaborations: Year-wise Trend by Type of Product, Since 2016
- Figure 9.6 Partnerships and Collaborations: Distribution by Type of Focus Area
- Figure 9.7 Most Active Players: Distribution by Number of Partnerships
- Figure 9.8 Partnerships and Collaborations: Distribution by Region (Continent-wise)
- Figure 9.9 Partnerships and Collaborations: Distribution by Region (Country-wise)
- Figure 10.1 Recent Expansions: Cumulative Year-wise Trend, Since 2016
- Figure 10.2 Recent Expansions: Distribution by Type of Expansion
- Figure 10.3 Recent Expansions: Distribution by Type of Expansion and Scale of Operation
- Figure 10.4 Recent Expansions: Distribution by Type of Product
- Figure 10.5 Recent Expansions: Distribution by Type of Expansion and Type of Product
- Figure 10.6 Recent Expansions: Distribution by Amount Invested (USD Million)
- Figure 10.7 Recent Expansions: Distribution by Company Size and Location of Headquarters
- Figure 10.8 Recent Expansions: Distribution by Location of Expanded Facility
- Figure 10.9 Recent Expansions: Distribution by Type of Expansion and Location of Expanded Facility
- Figure 10.10 Most Active Players: Distribution by Number of Recent Expansions
- Figure 10.11 Recent Expansions: Distribution by Year of Expansion and Region
- Figure 10.12 Recent Expansions: Distribution by Region (Country-wise)
- Figure 11.1. Lipid Contract Manufacturing Capacity: Distribution by Company Size
- Figure 11.2. Lipid Contract Manufacturing Capacity: Distribution by Scale of Operation
- Figure 11.3. Lipid Contract Manufacturing Capacity: Distribution by Location of Manufacturing Facility
- Figure 12.1. Make versus Buy Decision Making Framework
- Figure 12.2. Make versus Buy Decision Making: Description of Possible Scenarios
- Figure 13.1 Global Lipid Contract Manufacturing Market, Till 2035 (USD Billion)
- Figure 13.2 Lipid Contract Manufacturing Market, Till 2035: Distribution by Type of Product (USD Billion)
- Figure 13.3 Lipid Contract Manufacturing Market, Till 2035: Distribution by Company Size (USD Billion)
- Figure 13.4 Lipid Contract Manufacturing Market, Till 2035: Distribution by Scale of Operation (USD Billion)
- Figure 13.5 Lipid Contract Manufacturing Market in North America, Till 2035 (USD Billion)
- Figure 13.6 Lipid Contract Manufacturing Market in Europe, Till 2035 (USD Billion)
- Figure 13.7 Lipid Contract Manufacturing Market in Asia-Pacific, Till 2035 (USD Billion)
- Figure 13.8 Lipid Contract Manufacturing Market in MENA, Till 2035 (USD Billion)
- Figure 13.9 Lipid Contract Manufacturing Market in Latin America, Till 2035 (USD Billion)
- Figure 13.10 Lipid Contract Manufacturing Market in Rest of the World, Till 2035 (USD Billion)
- Figure 14.1 Biopharmaceutics Classification System (BCS)
- Figure 14.2 Types of Lipid-based Drug Delivery Systems
- Figure 14.3 Lipid-based mRNA Vaccines: Mechanism of Action
- Figure 15.1 Concluding Remarks: Overall Market Landscape
- Figure 15.2 Concluding Remarks: Partnerships and Collaborations
- Figure 15.3 Concluding Remarks: Recent Expansions
- Figure 15.4 Concluding Remarks: Capacity Analysis
- Figure 15.5 Concluding Remarks: Market Forecast










